Figure 6.
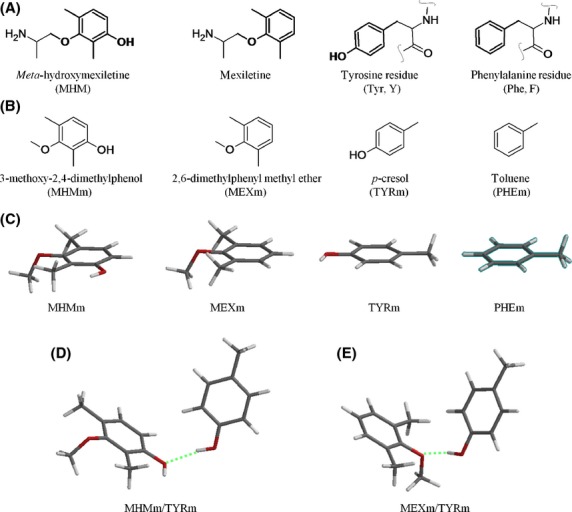
Structures of the model compounds used to predict the interaction energies between mexiletine and MHM with their putative binding aromatic residues – Tyr652 and Phe656. (A) guest and host molecules presenting in bold the moieties studied by ab initio calculations; (B) corresponding truncated molecules assumed as representative of the whole guest compounds and host aromatic residues; (C) tube representation of the most stable conformer [B3LYP/6-311++G(2df,2p)//B3LYP/6-311++G(2df,2p)] of each model compound undergoing complex formation; (D) complex formed by MHMm with TYRm, optimized at the above level of calculation (hydrogen bond shown as a green broken line); (E) complex formed by MEXm with TYRm, optimized at the above level of calculation (hydrogen bond shown as a green broken line).
