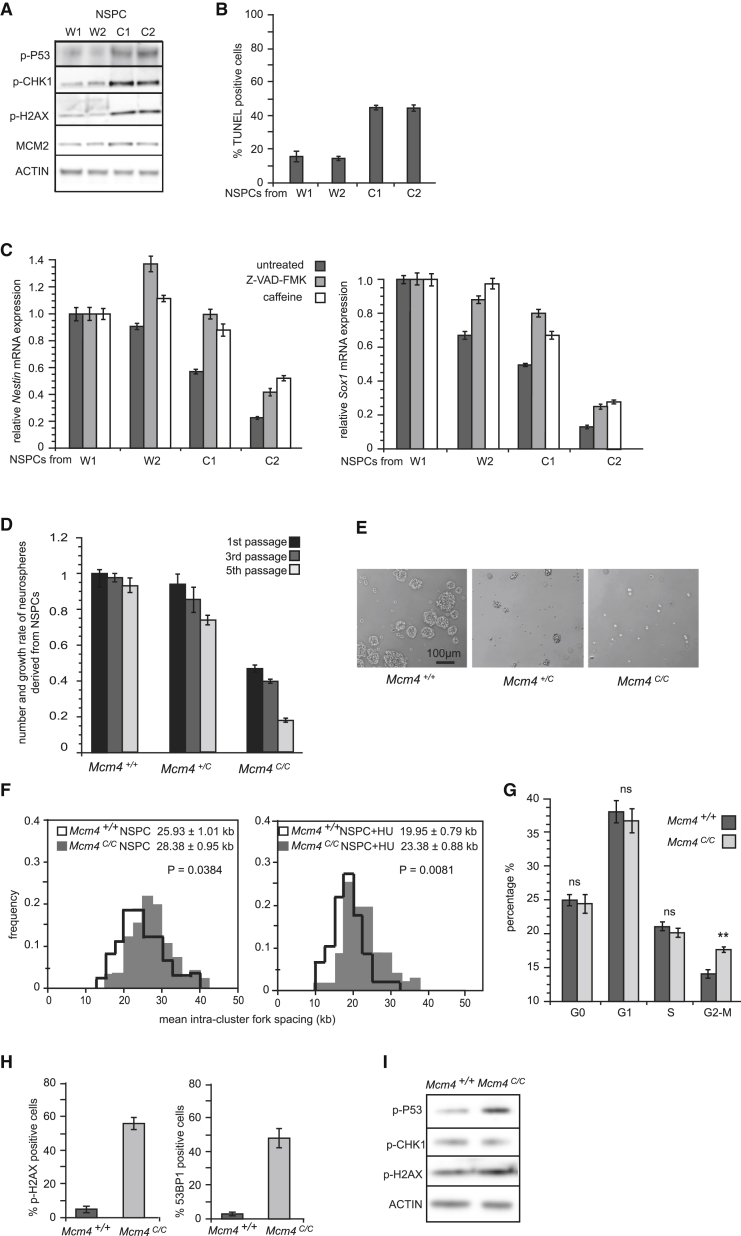
Figure 3.
Reducing DOs Impairs the Differentiation of NSPCs
(A–C) Analysis of the NSPC differentiation from the Mcm4+/+ (W1 and W2) and Mcm4C/C (C1 and C2) ESCs. (A) Immunoblot of the NSPC total lysate is shown. (B) TUNEL assay on NSPCs at 96 hr after induced differentiation is shown. (C) qRT-PCR of Nestin and Sox1 expression in NSPCs is shown. Treatment with caffeine (4 mM) or Z-VAD-FMK (40 μM) started at 48 hr after induction, and NSPCs were harvested at 96 hr for analysis.
(D–I) Analysis of neurospheres clonally derived from NSPCs isolated from the E13.5 mouse forebrain. (D) Neurospheres were passaged every 6 days to give a new round of clonogenic assay. Number and growth rate of neurospheres were measured by counting the neurospheres and the total number of cells at each passage. Error bars represent SEM from four independent experiments and each experiment containing five embryos of each genotype. (E) Representative images of neurospheres at fifth passage are shown. (F) DNA fiber analysis is shown. Cells were treated with 100 μM HU for 4 hr before analysis. Overall average fork spacing ± SEM from >50 replicon clusters is shown. p values are from two-tailed t test. (G) Cell-cycle analysis of neurospheres at fifth passage by FACS after pyronin Y and DAPI staining is shown. Note G2-M blockage of the cells in the Mcm4C/C neurospheres. Two-tailed t test: non-significant (ns); p < 0.005 (∗∗). (H) Immunofluorescence quantifying the percentage of γH2AX or 53BP1 positive cells in neurospheres is shown. (I) Immunoblot of total cell lysate of neurospheres is shown.
Error bars in (B), (C), (G), and (H) all represent SEM of three independent experiments. See also Figure S3.