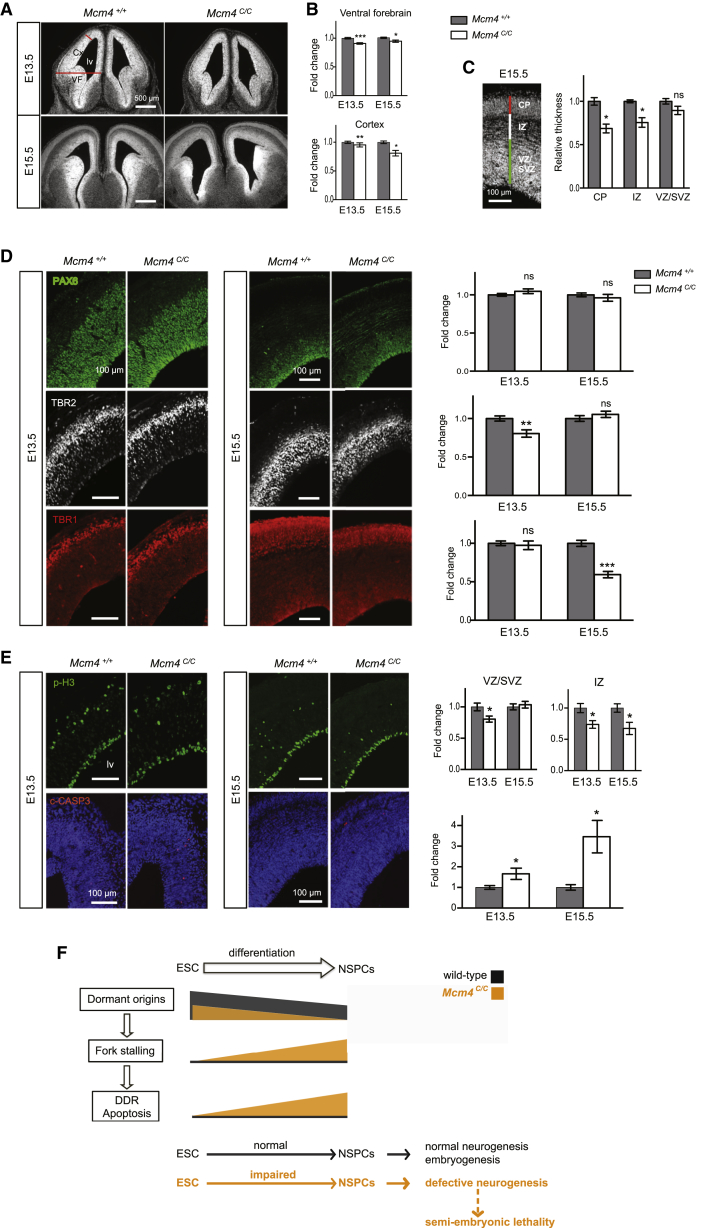
Figure 4.
Reducing DOs Impairs Embryonic Neurogenesis and Affects Embryonic Viability
(A and B) Coronal sections of the Mcm4+/+ and Mcm4C/C embryonic forebrain. (A) Phase contrast views are shown. Red lines show width of ventral forebrain (VF) and thickness of cortex (Cx). (B) Quantification of forebrain size is shown.
(C) DAPI staining and measurement of the E15.5 cortex: ventricular/sub-ventricular zone (VZ/SVZ); intermediate zone (IZ); and cortical plate (CP).
(D) Cortex coronal sections with immunolabeling of VZ/SVZ stem/progenitor cells (PAX6+), intermediate progenitor cells (TBR2+), and early born, deep-layer cortical neurons (TBR1+).
(E) Immunolabeling of phospho-H3 and cleaved-CASPASE 3 (c-CASP3) cells on cortex coronal sections. lv, lateral ventricles. Error bars represent SEM of three independent experiments comprising in total five Mcm4+/+ and four Mcm4C/C embryos at E13.5 and seven Mcm4+/+ and seven Mcm4C/C embryos at E15.5. Two-tailed t test: ns; p < 0.05 (∗); p < 0.005 (∗∗); p < 0.0005 (∗∗∗).
(F) Model. Black and orange colors indicate the conditions of the wild-type and the partial depletion of DOs (as in the Mcm4C/C mice), respectively. In the wild-type ESCs, DOs initiate back-up replication forks to rescue fork stalling and maintain genome integrity. ESCs possess a greater number of DOs than NSPCs. Upon reduction of DO, there is a further reduction of DOs in the NSPCs, likely reaching the threshold required to rescue the endogenous fork stalling during DNA replication. As a result, DNA damage is accumulated and cell death incurs, eventually impairing NSPC proliferation and differentiation. This explains the neurogenic defect in the Mcm4C/C embryos, which could contribute to the semi-embryonic lethality of the Mcm4C/C mice.
See also Figure S4.