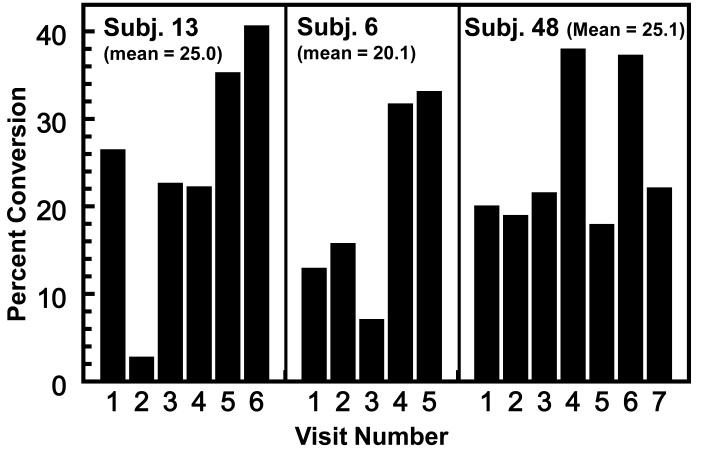
Figure 4.
Consistency of conversion of GR to urinary ITC in 3 high-converter phenotypes who were tested on 5-7 occasions (selected from the 131 determinations shown in Fig. 1A). Each determination involved a single oral administration of 200 μmol of GR and measurement of 24-h DTC excretion. The results are expressed as percent of administered dose. The intervals between tests varied from a few days to 2.5 years.