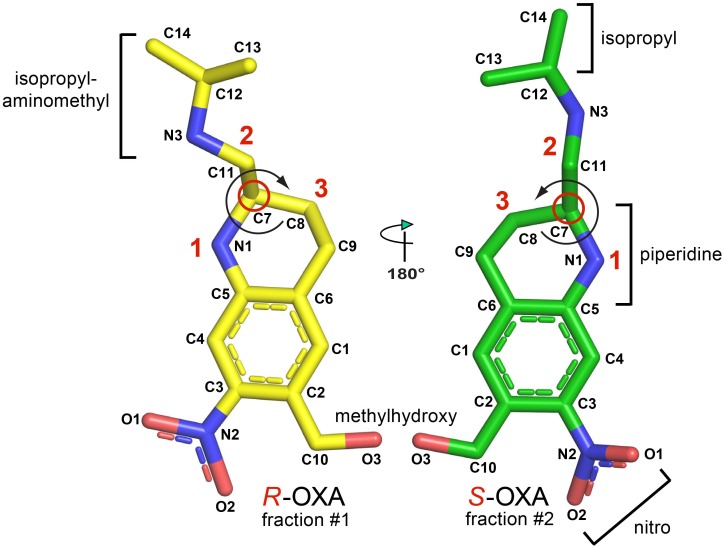
Fig 1. R-oxamniquine and S-oxamniquine indicating the functional groups and the chiral carbons (red circles).
The absolute configurations are determined by the substituents coming off of the chiral carbon atom using the Cahn-Ingold-Prelog rules. The molecules are rotated such that the lowest priority substituent (H-atom) is in the back of the chiral carbon, facing away from the viewer. Curves are drawn from substituents 1 to 2 to 3 substituent. If two substituents have the same immediate substituent atom, evaluate atoms progressively further away from the chiral center until a difference is found. If the curve is clockwise, the stereocenter is of R-configuration. If the curve is counterclockwise, the stereocenter is of S-confguration.