Fig. 6.
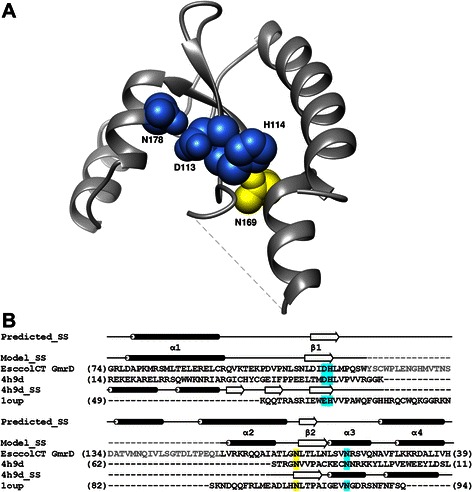
Structural models for the catalytic core of the GmrD domain. a The protein backbone is shown in the ribbon representation. Predicted functionally important residues are labeled and shown in the space-filled representation. Residues predicted to be involved in catalysis are colored in blue, N169 predicted to fulfill a crucial structural role – in yellow. The dashed line indicates the location of the long insertion removed from the model. The coordinates are available from ftp://genesilico.pl/iamb/models/GmrSD/GmrD.pdb. b Target-template alignment used for comparative modeling of the GmrD domain from the GmrSD CT enzyme. The sequence of Vvn endonuclease [PDB:1OUP] is included in the alignment to indicate the active site residues, although this structure was not used as a template for comparative modeling. Consensus secondary structure calculated by the GeneSilico metaserver (“Predicted_SS”) and secondary structure of the GmrD model (“Model_SS”) are indicated above the alignment as tubes (helices) and arrows (strands). Numbering of the secondary structure elements refers to the model. Secondary structure of the template used for modeling (Mn-dependent Gme HNH nicking endonuclease from Geobacter metallireducens GS-15, [PDB:4H9D]) is indicated below the template sequence. Residues predicted to be involved in catalysis are marked in blue, the Asn residue predicted to fulfill a crucial structural role – in yellow. The sequence of the long insertion not included in the model is marked in grey letters
