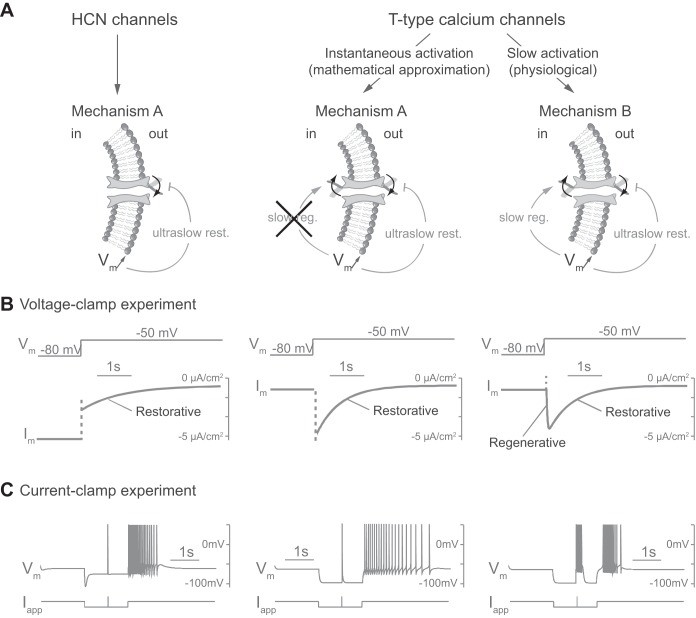
Fig. 2.
Slow activation of T-type calcium channels is the distinctive difference between the PIR mechanisms A and B. A: schemes representing ion channel gating in different cases. HCN, hyperpolarization-activated cyclic nucleotide-gated channels. B: voltage-clamp experiment in silico. Responses of transmembrane current (Im) to a varying externally applied potential (Vm, varies from −80 to −50 mV) for each case. C: current-clamp experiment in silico. Responses of membrane potential (Vm) to a varying external applied current (Iapp) for each case. The applied current is identical to that in Fig. 1 but takes a value of 10 μA/cm2 for 10 ms for the fast depolarizing input. Both mechanisms trigger a PIR via an ultraslow inward current in response to hyperpolarization, which brings ultraslow restorativity (ultraslow rest.) to the neuron (see materials and methods for a description of cellular models and applied currents). In addition, T-type calcium channels in mechanism B, because of their slow activation, are a source of slow regenerativity (slow reg.). An instantaneous activation of the T-type calcium channels, i.e., steady-state approximation of their activation, suppresses this slow regenerativity and produces a mechanism A PIR. The mechanism B PIR is endogenous, as revealed by the specific signature during hyperpolarization.