Abstract
Definitive endoderm (DE) is a vital precursor for internal organs such as liver and pancreas. Efficient protocol to differentiate human embryonic stem cells (hESCs) or induced pluripotent stem cells (iPSCs) to DE is essential for regenerative medicine and for modeling diseases; yet, poor cell survival during DE differentiation remains unsolved. In this study, our use of B27 supplement in modified differentiation protocols has led to a substantial improvement. We used an SOX17-enhanced green fluorescent protein (eGFP) reporter hESC line to compare and modify established DE differentiation protocols. Both total live cell numbers and the percentages of eGFP-positive cells were used to assess differentiation efficiency. Among tested protocols, three modified protocols with serum-free B27 supplement were developed to generate a high number of DE cells. Massive cell death was avoided during DE differentiation and the percentage of DE cells remained high. When the resulting DE cells were further differentiated toward the pancreatic lineage, the expression of pancreatic-specific markers was significantly increased. Similar high DE differentiation efficiency was observed in H1 hESCs and iPSCs through the modified protocols. In B27 components, bovine serum albumin was found to facilitate DE differentiation and cell survival. Using our modified DE differentiation protocols, satisfactory quantities of quality DE can be produced as primary material for further endoderm lineage differentiation.
Introduction
Generation of lineage-specific cells for cell replacement therapy is one of the ultimate goals in regenerative medicine [1,2]. Pluripotent stem cells (PSCs), such as human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs), can differentiate into all three germ layers: definitive endoderm (DE), mesoderm, and ectoderm [3,4]. Internal organs, including the pancreas, liver, lungs, thyroid, and thymus, are derived from the endoderm lineage. The generation of DE, the first step in PSC differentiation, is extremely critical to obtain mature and fully functional endoderm lineages [5]. Various protocols have been developed to generate DE in vitro by recapitulating in vivo embryogenesis, but their efficiency varies widely [6–11]. It has been reported that the initiating DE density is influential for the final differentiation yield [12]. In view of the variability in DE production outcomes, validation and improvement of these protocols are needed.
Sox17, a high-mobility-group box domain (HMG domain) transcription factor, is essential for DE formation [13,14]. SOX17 has been widely used as a DE-specific marker for hESC-derived DE [15,16]. In DE differentiation studies, the percentage of DE varies from protocol to protocol; thus, the SOX17 gene expression level used for assessment of DE differentiation reflects a heterogeneous population, not just DE. As a relative value, it is hard to compare this parameter among studies. Additionally, this parameter does not always represent the exact differentiation efficiency due to the heterogeneity of differentiation products. Accurate and quantitative methods are needed for comparing protocols. The establishment of a reporter hESC line with an enhanced green fluorescent protein (eGFP) targeted to the SOX17 locus offers a valuable tool for in vitro endodermal development analysis [17]. In these SOX17-eGFP cells, eGFP expression was reported to faithfully represent SOX17-expressing endoderm cells [17]. With this cell line, an accurate and quantitative examination of DE differentiation through eGFP expression is available. Therefore, in this study, such an SOX17-eGFP reporter cell line was used to monitor the progress of DE differentiation and to assess DE differentiation protocols.
In previous studies, DE differentiation was reported to reach as high as 80% [15,18]. However, in the first stage of in vitro hESC differentiation, dramatic cell losses were observed in our previous study (unpublished data). This high level of cell loss leads to a small absolute DE cell number in spite of a high DE percentage in the final product. No study has been done to either assess DE efficiency with consideration of the final total live cell number or improve cell survival during DE differentiation.
Collectively, to have a thorough evaluation of differentiation efficiency, measurement of both the percentage of differentiated DE cells and the total live cell number is necessary. In this study, we used an SOX17-eGFP hESC reporter cell line and both aforementioned parameters (final total live cell number and the percentage of eGFP+ cells) to compare and optimize DE differentiation protocols. Over 40 protocols, including 7 published ones, were compared and 3 optimized protocols were developed as a result. These optimized protocols were also effective in supporting differentiation of other hESCs and iPSCs toward DE. The progenies from these protocols favored pancreatic lineage differentiation. B27, a key component in all three optimized protocols, was identified to improve cell survival during DE differentiation. As a result of this study, satisfactory quantities of quality DE cells can be produced and used as primary material for further endoderm lineage differentiation.
Materials and Methods
Human iPSC generation
Three oriP/EBNA-based episomal vectors (obtained from Addgene), pEP4EO2SEN2K, pEP4EO2SET2K, and pCEP4-M2L, were cotransfected into 2×106 human newborn fibroblasts (ATCC® CRL-2703) through nucleofection using the Amaxa 4D-nucleofector system. The transfected fibroblasts were placed onto Matrigel-coated dishes in human fibroblast medium [Dulbecco's modified Eagle's medium (DMEM), 10% fetal bovine serum (FBS), 2 mM l-glutamine, 50 units/mL penicillin, and 50 mg/mL streptomycin]. On day 1 post-transfection, the fibroblast medium was replaced with hESC medium (GlobalStem; Molecular Transfer, Inc.) supplemented with 20% knockout serum replacer (Life Technologies) and 0.5 mM sodium butyrate (Sigma-Aldrich). The culture medium was refreshed every other day. Around day 20, visible colonies with ESC-like morphology were picked and transferred to mouse embryonic fibroblast feeder layers in hESC medium supplemented with 20 ng/mL of basic fibroblast growth factor (bFGF; Life Technologies).
hESC and iPSC culture
hESC lines, S17d5 and H1, as well as iPSCs, were cultured on γ radiation-inactivated mouse embryonic fibroblasts. Cell culture medium was replaced daily. The medium comprised 80% DMEM/F12 (11330-032; Life Technologies), 20% knockout serum replacer (10828-028; Life Technologies), 1 mM l-glutamine (35050-061; Life Technologies), 0.1 mM 2-mercaptoethanol (21985-023; Life Technologies), 0.1 mM nonessential amino acids (11140-050; Life Technologies), and 16 ng/mL recombinant bFGF (233-FB/CF; R&D Systems). After confluency, cells were pretreated with 10 μM ROCK inhibitor Y27632 (CD0141; Chemdea), dissociated by Accutase (SCR005; Millipore), and seeded with 10 μM Y27632 in fresh culture medium. The undifferentiated cells were passaged every 3–5 days in a ratio of 1:3 to 1:5.
Feeder-free culture of S17d5 cells was performed on 8.8 μg/cm2 Matrigel (354230; Corning) in mTeSR1 media (05850; Stem Cell Technologies). The subculture procedure, ratio, and frequency for feeder-free culture were the same as the aforementioned feeder culture.
Cell differentiation
The DE differentiation was performed using seven published protocols as described elsewhere (Fig. 1A) [6–11]. The DE differentiation basal medium in the modified D'Amour (Mod-D'Amour) protocol and three respective modified protocols (Protocol 7, Protocol 4, and Combined Protocol 4 and 7) was R medium, which comprised 1% nonessential amino acids, 2 mM l-glutamine, 50 units/mL of penicillin, and 50 μg/mL of streptomycin (P4333; Sigma-Aldrich) in RPMI-1640 medium (R8758; Sigma-Aldrich) (Fig. 2A).
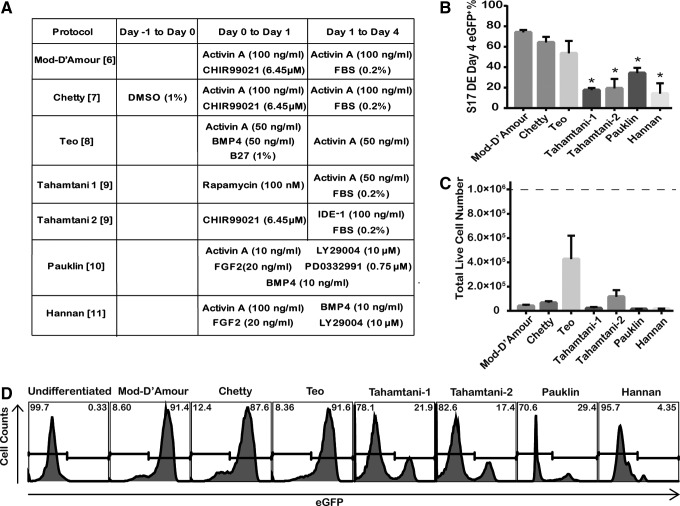
FIG. 1.
Comparison of seven published protocols for definitive endoderm (DE) differentiation. (A) Summary of published DE differentiation protocols [6–11]. In the modified D'Amour (Mod-D'Amour), Chetty, Teo, Tahamtani 1, and Tahamtani 2 protocols, growth factors, small molecules, and supplements were added to R medium (comprising 1% nonessential amino acids, 2 mM l-glutamine, 50 units/mL of penicillin, and 50 μg/mL of streptomycin in RPMI-1640 medium) in a two-step manner. In the Pauklin and Hannan protocols, chemically defined medium containing polyvinyl alcohol was used as the basal medium. FBS, fetal bovine serum; BMP4, bone morphogenetic protein 4; FGF2, fibroblast growth factor 2. (B) The percentage of eGFP+ cells after 4-day DE differentiation of S17d5 cells. (C) Total live cell numbers at the end of 4-day DE differentiation of S17d5 cells. Independent runs were normalized to 1.0×106 starting undifferentiated cells (dotted line). *P<0.05 compared with the Mod-D'Amour protocol by Student's unpaired t-test. Data are presented as mean±standard error of the mean (SEM), n=3–5. (D) Representative flow cytometric histograms of SOX17-enhanced green fluorescent protein (eGFP) induction through published DE differentiation protocols.
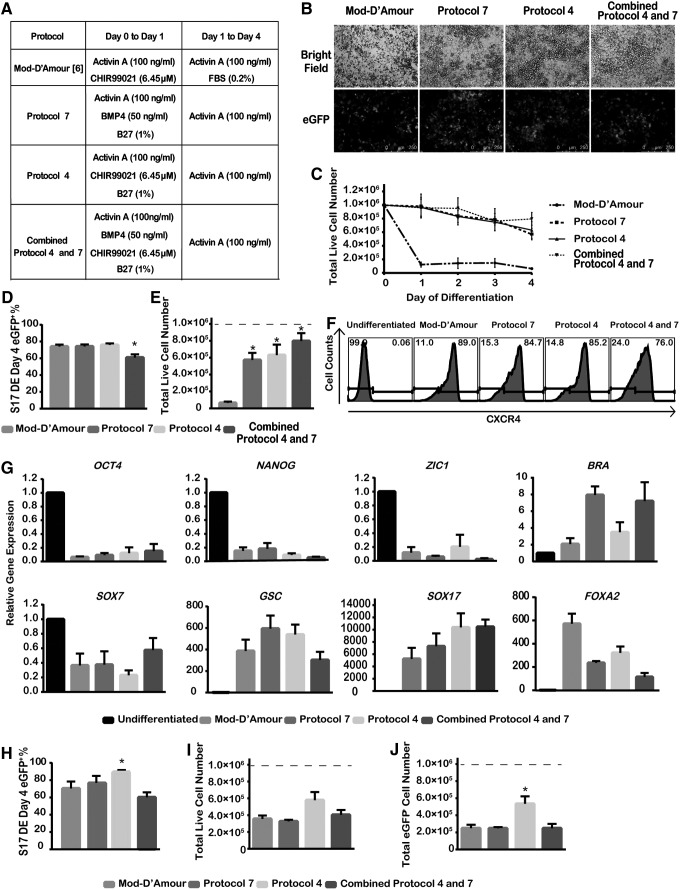
FIG. 2.
Three modified protocols for DE differentiation. (A) Summary of control and three modified DE differentiation protocols. Supplemented R medium (comprising 1% nonessential amino acids, 2 mM l-glutamine, 50 units/mL of penicillin, and 50 μg/mL of streptomycin in RPMI-1640 medium) was added to induce differentiation on a daily basis. (B) Bright field and immunofluorescence of eGFP+ cells on day 4 of S17d5 cell DE differentiation. Scale bar, 250 μm. (C) Daily total live cell number changes during S17d5 cell DE differentiation. (D) The percentage of eGFP+ cells after 4-day DE differentiation of S17d5 cells. (E) Total live cell numbers at the end of 4-day DE differentiation of S17d5 cells. (F) Representative flow cytometric histograms of CXCR4 induction after 4-day DE differentiation of S17d5 cells. (G) Gene expression of lineage-specific markers on day 4 of DE differentiation. OCT4 and NANOG, pluripotency markers; ZIC1, ectoderm marker; Brachyury (BRA), mesoderm marker; SOX7, visceral endoderm marker; GSC, mesendoderm marker; SOX17 and FOXA2, endoderm markers. (H–J) Three modified protocols for DE differentiation in feeder-free condition. Independent runs were normalized to 1.0×106 starting undifferentiated cells (dotted line). *P<0.05 compared with the Mod-D'Amour protocol by Student's unpaired t-test. Data are presented as mean±SEM, n=3–5.
When the density of undifferentiated cells reached 3.2–5.2×105 cells/cm2, differentiation was initiated, and this differentiation initiating day was counted as day 0. On day 0, the Mod-D'Amour protocol included 100 ng/mL activin A and 6.45 μM CHIR99021 (13122; Cayman Chemical) in R medium; Protocol 7 included 100 ng/mL activin A, 50 ng/mL bone morphogenetic protein 4 (BMP4), and 1% B27 (17504-044; Life Technologies); Protocol 4 included 100 ng/mL activin A, 6.45 μM CHIR99021, and 1% B27; and the Combined Protocol 4 and 7 included 100 ng/mL activin A, 50 ng/mL BMP4, 6.45 μM CHIR99021, and 1% B27 (Fig. 2A). In the subsequent 3 days of DE differentiation, the Mod-D'Amour protocol was supplemented with 100 ng/mL activin A and 0.2% FBS (16000-044; Life Technologies), while the three modified protocols (Protocols 4, 7, and 4+7) were supplemented with only 100 ng/mL activin A (Fig. 2A). After DE differentiation, from day 4, S17d5 cells were continuously differentiated toward insulin-producing cells (IPCs) following previously described protocols [19].
All growth factors were purchased from R&D Systems. In the B27 component study, the use of CHIR99021 and activin A was the same as in Protocol 4, while the use of 1% B27 (17504-044; Life Technologies) in Protocol 4 on day 0 was replaced with 1% B27 without vitamin A (12587-010; Life Technologies), 1% B27 without antioxidants (10889-038; Life Technologies), 1% bovine serum albumin (BSA, fatty acid-free, 03117057001; Roche), or 1% N2 (17502-048; Life Technologies), respectively. The details of all other tested protocols are listed in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/scd).
Flow cytometry
Cells were dissociated with Accutase and prepared as single-cell suspensions. For analysis of the percentage of eGFP+ cells (eGFP+%), each single-cell suspension was directly analyzed. For analysis of the percentage of CXCR4+ cells (CXCR4+%) in S17d5 and H1 hESCs and iPSCs, single-cell suspensions were stained with phycoerythrin-conjugated anti-human CXCR4 antibody (1/80, 306506; BioLegend) and analyzed. A viability dye, 7-amino-actinomycin D (420404; BioLegend) at 0.5 μg/mL, was used to stain each sample before flow cytometric analysis to eliminate background noise from dead cells. Flow cytometric analysis was performed with a BD LSRII Flow Cytometer and analyzed by FACSDiva software.
Quantitative reverse transcriptase–polymerase chain reaction
Total RNA was isolated from cells with a Direct-zol RNA MiniPrep kit (R2052; Zymo Research) and used to perform reverse transcription with the High-Capacity cDNA Reverse Transcription kit (4368814; Applied Biosystems). Quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) was performed in duplicate using iTaq Universal SYBR Green Supermix (1725120; Bio-Rad) and the Bio-Rad CFX96 Real-Time System. Expression levels were normalized using actin as an endogenous control.
Primer sequences used in qRT-PCR were as follows: ACTB (forward, 5′-CATGTACGTTGCTATCCAGGC-3′, and reverse, 5′-CTC CTTAATGTCACGCACGAT-3′), OCT4 (forward, 5′-GGG AGATTGATAACTGGTGTGTT-3′, and reverse, 5′-GTGTA TATCCCAGGGTGATCCTC-3′), NANOG (forward, 5′-TTT GTGGGCCTGAAGAAAACT-3′, and reverse, 5′-AGGGC TGTCCTGAATAAGCAG-3′), ZIC1 (forward, 5′-CGTTCG GAGCACTATGCTG-3′, and reverse, 5′-TGTTGCACGAC TTTTTGGGGT-3′), Brachyury (BRA) (forward, 5′-TATG AGCCTCGAATCCACATAGT-3′, and reverse, 5′-CCTCG TTCTGATAAGCAGTCAC-3′), SOX7 (forward, 5′-ACGC CGAGCTCAGCAAGAT-3′, and reverse, 5′-TCCACGTA CGGCCTCTTCTG-3′), GSC (forward, 5′-AACGCGGAGA AGTGGAACAAG-3′, and reverse, 5′-CTGTCCGAGTCCA AATCGC-3′), SOX17 (forward, 5′-GGCGCAGCAGAATC CAGA-3′, and reverse, 5′-CCACGACTTGCCCAGCAT-3′), FOXA2 (forward, 5′-GGGAGCGGTGAAGATGGA-3′, and reverse, 5′-TCATGTTGCTCACGGAGGAGTA-3′), insulin (INS) (forward, 5′-AAGAGGCCATCAAGCAGATCA-3′, and reverse, 5′-CAGGAGGCGCATCCACA-3′), PDX1 (forward, 5′-AAGTCTACCAAAGCTCACGCG-3′, and reverse, 5′-GTAGGCGCCGCCTGC-3′), chromogranin A (CHGA) (forward, 5′-TAAAGGGGATACCGAGGTGATG-3′, and reverse, 5′-TCGGAGTGTCTCAAAACATTCC-3′), AFP (forward, 5′-CTTTGGGCTGCTCGCTATGA-3′, and reverse, 5′-GCATGTTGATTTAACAAGCTGCT-3′), ALB (forward, 5′-TGCAACTCTTCGTGAAACCTATG-3′, and reverse, 5′-ACATCAACCTCTGGTCTCACC-3′), and CDX2 (forward, 5′-GACGTGAGCATGTACCCTAGC-3′, and reverse, 5′-GCG TAGCCATTCCAGTCCT-3′).
Total live cell number counts
Cells were dissociated with Accutase into single-cell suspensions. Trypan blue at 0.2% was mixed with single-cell suspensions in a 1:1 ratio, and the live cell density was counted with a Cellometer Mini (Nexcelom Bioscience). The total live cell number was calculated by multiplying the volume of a single-cell suspension by the live cell density. The results were normalized to 1 million starting undifferentiated cells on day 0. The total eGFP+ cell number was calculated by multiplying the percentage of eGFP+ cells by the total live cell number.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde at 25°C for 15 min, washed with phosphate-buffered saline (PBS), and blocked with 5% donkey serum for 1 h in PBS. After blocking, 10 μg/mL human SOX17 antibody (AF1924; R&D Systems) was used as a primary antibody with incubation overnight at 4°C, and a donkey anti-goat secondary antibody (1/250; 705-605-147; Jackson ImmunoResearch Laboratories) was applied at 25°C for 1 h. Cells were washed thrice with PBS and counterstained with DAPI. Photography was done using a Leica DMI6000 B inverted microscope with Leica Application Suite Advanced Fluorescence software.
Statistical analysis
The analysis was done on three to five independent experiments. Results are presented as mean±standard error of the mean. Student's unpaired t-test was used for comparing two datasets. Differences with P values smaller than 0.05 (P<0.05) were considered statistically significant. GraphPad Prism software was used in all statistical analysis.
Results
Comparing published DE differentiation protocols
To find the most efficient DE differentiation strategy, we first performed a systematic comparison of published DE differentiation protocols with the SOX17-eGFP reporter hESC line, S17d5. The ability of S17d5 to identify SOX17+ DE cells was assessed by differentiating cells with the first reported protocol established by D'Amour et al. [6]. Due to the low bioactivity and high expense of using Wnt3a recombinant protein, it was replaced by a glycogen synthase kinase-3 (GSK-3) inhibitor, CHIR99021, in the D'Amour protocol [20]. This modified D'Amour protocol was termed Mod-D'Amour and used as a control while testing different modified protocols. In undifferentiated hESCs, more than 99% of cells were eGFP−, while after 4 days of differentiation, 74.0%±2.3% of cells became eGFP+. Immunostaining of S17d5 cells showed that on day 4, 100% SOX17+ cells were eGFP+ and 100% eGFP+ cells were SOX17+. These data confirmed a previous study showing that eGFP expression faithfully represents SOX17 expression in this reporter cell line [17].
We next used S17d5 cells to evaluate the efficiency of seven published DE differentiation protocols. Various growth factors, small molecules, supplements, and basal media were applied in these protocols (Fig. 1A) [6–11]. Two parameters were used for evaluation: the total live cell number and eGFP+%. The Mod-D'Amour, Chetty, and Teo protocols resulted in a similar high eGFP+% of over 50%, while the Tahamtani-1, Tahamtani-2, Pauklin, and Hannan protocols led to a significantly lower eGFP+% than the Mod-D'Amour protocol (Fig. 1B). Representative flow cytometric histograms of eGFP fluorescence are shown in Fig. 1D. We observed that abundant cell death during the differentiation process led to a low total live cell number on day 4 in all the tested protocols (Fig. 1C). Among these seven protocols, the Teo protocol produced the most live cells; however, the cell number was low, suggesting that optimization is still required.
Optimizing DE differentiation protocols
From the seven published protocols, different components (such as small molecules, growth factors, and basal media) were selected to generate 26 distinct combinations, which were tested for DE differentiation (Supplementary Table S1), including three protocols that were modified from the Teo protocol (Protocol 7, Protocol 4, and Combined Protocol 4 and 7). The Teo protocol was identified as the most efficient among all the published protocols tested. The differences between the Teo protocol and its respective control Mod-D'Amour, included half the concentration of activin A, the use of BMP4, and the replacement of low concentration (0.2%) FBS by B27 supplements. Among the 26 modified protocols, we found that the three protocols modified from the Teo protocol were the most robust protocols (Fig. 2A) on endoderm differentiation. Live eGFP+ cells were observed directly under a fluorescence microscope to provide an immediate quality control for DE differentiation efficiency (Fig. 2B).
Percentages of eGFP+ cells and the total live cell number on day 4 of differentiation were assessed quantitatively. Compared with the Mod-D'Amour eGFP+%, Protocol 7 and Protocol 4 generated similar high eGFP+ percentages (74.1%±2.4% and 76.0%±1.9%, respectively), while Combined Protocol 4 and 7 generated 60.9%±3.9%, which was significantly lower (Fig. 2D). However, the total live cells increased by 8.9- to 12.4-folds in the modified protocols (Fig. 2E). No synergistic effect was observed from Combined Protocol 4 and 7 versus Protocol 4 or 7. From the daily live cell count, the major cell death in the Mod-D'Amour protocol occurred on the first day of differentiation (Fig. 2C). However, supplementation with 1% of B27 prevented massive cell death. To validate our findings from SOX17-eGFP expression, flow cytometric analysis with an additional endoderm marker, CXCR4, was used to label differentiated cells on day 4 (Fig. 2F). Consistent with the eGFP results, similar percentages of CXCR4+ cells were observed from products of both the Mod-D'Amour and the modified protocols.
To assess gene expression, we performed qRT-PCR to measure marker genes for pluripotency, ectoderm, mesoderm, and endoderm in day-4 differentiated cells (Fig. 2G). Expression of pluripotent markers, OCT4 and NANOG, the ectoderm marker, ZIC1, and the visceral endoderm marker, SOX7, was low. Protocol 7 and the combination Protocol 7 and 4 increased mesoderm marker BRA expression compared with the other protocols. Levels of the mesendoderm marker, GSC, and the endoderm marker, FOXA2, increased by over 100-fold in all protocols. Expression of the key endoderm marker, SOX17, increased more than 1,000-fold in all protocols. Our data suggest that the three modified protocols are optimized to prevent massive cell death during endoderm differentiation and are able to generate DE cells both at high percentages and in large amounts.
Feeder-free culture of hESCs and iPSCs has increased in popularity. To determine the DE differentiation efficiency of three modified protocols in feeder-free condition, undifferentiated S17d5 was transferred onto Matrigel in mTeSR1 and passaged thrice to adapt to the condition. We then performed DE differentiation and observed less cell death in feeder-free culture than on feeders for the Mod-D'Amour protocol during differentiation (Fig. 2E, I). One possible reason for this difference is that the adaptation to feeder-free culture may increases stress tolerance of hESCs [21]. When comparing the three modified protocols, the total live cell number did not show a significant difference (Fig. 2I). However, Protocol 4 consistently has eGFP+ cells at a higher percentage (92.6%±1.0%) compared with Mod-D'Amour (70.4%±8.0%) and generated more than 2-fold of total GFP+ cells as a result (Fig. 2H, J). To summarize, on the first day in feeder-free culture condition, the addition of B27 was beneficial for high efficient DE induction and produced more than twice the DE cells compared with the Mod-D'Amour protocol.
Differentiation toward the pancreatic lineage
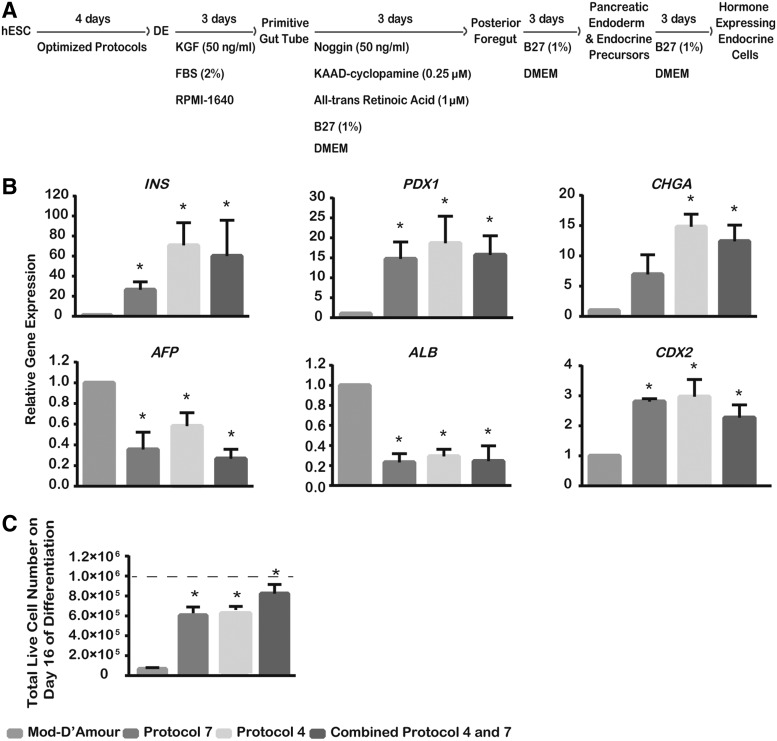
One major clinical application of in vitro produced DE cells is to further differentiate into IPCs. To determine whether DE cells generated through modified protocols can efficiently differentiate into pancreatic progenitors/IPCs, S17d5 cells were further differentiated through a stepwise procedure (Fig. 3A). Four steps were performed as previously reported: DE to primitive gut tube, posterior foregut, pancreatic endoderm and endocrine precursors, and finally hormone-expressing endocrine cells [19]. The progenies from different DE protocols were evaluated by the expression of lineage-specific markers. Compared with the Mod-D'Amour protocol, the gene expression of the IPC-specific markers, CHGA, INS, and PDX1, was significantly increased in progenies from the three optimized DE protocols (Protocols 4, 7, and 4+7) (Fig. 3B). In the meantime, the expression of the hepatic-specific markers, ALB and AFP, was significantly downregulated. Progenies from the three optimized DE protocols (Protocols 4, 7, and 4+7) generated pancreatic lineages as efficiently as, or even better than, the progenies from the Mod-D'Amour DE protocol. Moreover, no significant cell loss was observed in the process of IPC differentiation after DE formation. We assessed the total live cell number on day 16 of IPC differentiation. As shown in Fig. 3C, the total live cell number on day 16 was maintained at the level of day 4 (Figs. 2E and 3C).
FIG. 3.
DE cells generated through optimized protocols efficiently produce pancreatic progenitors/insulin-producing cells (IPCs). (A) Schematic of IPCs differentiated through optimized DE protocols subsequent to the pancreatic hormone–expressing endocrine cell differentiation protocol [19]. KGF, keratinocyte growth factor; DMEM, Dulbecco's modified Eagle's medium. (B) Gene expression of endoderm lineage-specific markers on day 16 of cell differentiation. The relative expression of markers by Mod-D'Amour progenies was set as 1, whereas other protocols were compared with Mod-D'Amour. INS, PDX1, and CHGA, pancreatic markers; ALB and AFP, hepatic-specific makers; CDX2, intestinal marker. (C) Total live cell numbers at the end of 16-day IPC differentiation of S17d5 cells. Independent runs were normalized to 1.0×106 starting undifferentiated cells (dotted line). *P<0.05 compared with the Mod-D'Amour protocol by Student's unpaired t-test. Values presented are mean±SEM, n=3–5.
Thus, our results suggest that DE produced by the modified protocols can be efficiently differentiated toward pancreatic lineages with much higher cell numbers. Application of these three optimized DE protocols may be able to increase the efficiency of efforts aimed at pancreatic lineage differentiation by providing more DE cells at the onset of differentiation.
Optimized DE protocols enhance DE differentiation from other hESCs and iPSCs
Different pluripotent cell lines were found to have varying differentiation potentials [22,23]. To confirm that our modified DE differentiation protocols can be used on other pluripotent cells, additional cell lines were tested, including the hESC line, H1, and an iPSC line. The total live cell number was assessed at the end of the 4-day differentiation process. Similar to S17d5 cells, the total live cell numbers increased significantly in the modified protocols compared with the Mod-D'Amour protocol in both H1 hESCs and iPSCs (Supplementary Figs S1A and S2A). After differentiation, cells were analyzed by flow cytometry with the endoderm marker, CXCR4 (Supplementary Figs S1B and S2B). The percentages of CXCR4+ cells from both H1 hESCs and iPSCs were similar among the Mod-D'Amour protocol and the three modified protocols. The percentage of CXCR4+ endoderm cells derived from iPSCs was slightly lower in all the protocols compared with H1. This may be due to a lower endoderm differentiation potential of this iPSC line. The success of DE differentiation of H1 hESCs and iPSCs was also shown by a decreased expression of pluripotent markers, OCT4 and NANOG, and increased expression of DE markers, SOX17 and FOXA2 (Supplementary Figs S1C and S2C). These data suggest that the three modified protocols can optimize the generation of DE cells in other hESCs and iPSCs.
B27 components facilitate DE differentiation and sustain cell survival
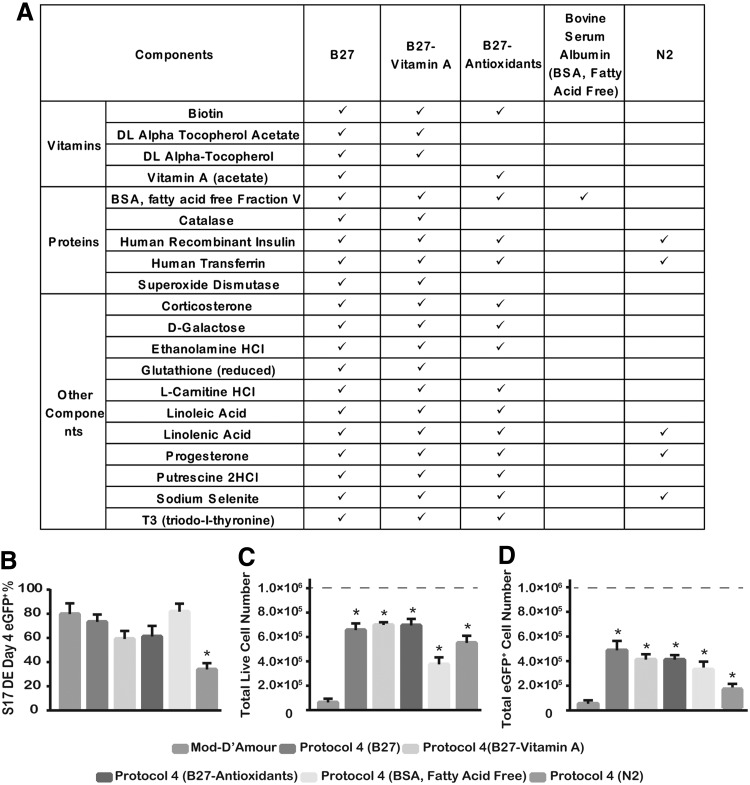
In the three modified protocols, the use of B27 supplement in the first day of differentiation was shown to improve cell survival during DE differentiation while maintaining differentiation efficiency. To identify which component in B27 contributed to this effect, four different supplements with partial B27 components were used to replace complete B27 in Protocol 4 during DE differentiation. These replacements included B27 without vitamin A (B27-Vitamin A), B27 without antioxidant (B27-Antioxidants), BSA (fatty acid-free), and N2 (Fig. 4A). Compared with Mod-D'Amour and Protocol 4, the substitution of B27 by B27-Vitamin A, B27-Antioxidants, and BSA fatty acid-free in Protocol 4 generated similar high eGFP+ percentages (59.2%±6.5%, 61.2%±8.7%, and 81.8%±6.6%, respectively), but not N2 supplement (34.0%±5.2%) (Fig. 4B). One-day treatment of all of these B27 substitutes was able to generate significantly higher total live cell numbers and eGFP+ cell numbers compared with 3-day FBS (0.2%) treatment in the Mod-D'Amour protocol (Fig. 4C, D). BSA alone showed a great improvement on eGFP+ cell numbers, although it was not as good as complete B27. Interestingly, N2 supplement increased the total live cell number, but reduced eGFP+% (Fig. 4B, C). These data suggest that BSA (fatty acid-free) may be the major component in B27 to improve DE differentiation.
FIG. 4.
Substituting B27 for other supplements in Protocol 4. (A) Formulation comparison of supplements. (B) The percentage of eGFP+ cells after 4-day DE differentiation of S17d5 cells. (C) Total live cell numbers at the end of 4-day DE differentiation of S17d5 cells. (D) Total live eGFP+ cell numbers at the end of 4-day DE differentiation of S17d5 cells. Independent runs were normalized to 1.0×106 starting undifferentiated cells (dotted line). *P<0.05 compared with the Mod-D'Amour protocol by Student's unpaired t-test. Data are presented as mean±SEM, n=3–4.
Discussion
DE is the first stage of in vitro differentiation from PSCs to vital organ lineages with therapeutic potential, such as pancreatic beta cells and hepatocytes. The combination of activin A and Wnt3a has been commonly used to induce DE differentiation. High percentages of DE cells, up to 80%–90%, can be achieved through such treatments [15,18]. However, poor cell survival occurred during this induction [24]. To overcome the high level of cell death, optimization of the DE differentiation procedure is needed. In this study, we used an SOX17-eGFP reporter hESC line to assess previously developed DE differentiation protocols and to modify protocols for a higher cell survival rate. The differentiation into DE was directly observed through SOX17-eGFP expression. Over 40 combinations involving different components were tested (Supplementary Table S1). We found that four factors are important in inducing DE differentiation and maintaining cell survival: activin A, the GSK-3 inhibitor CHIR99021, BMP4, and B27 supplement.
The role of activin A in stimulation of the transforming growth factor beta signaling pathway and induction of DE differentiation has been widely recognized [25,26]. A concentration of 10–100 ng/mL of activin A has been applied in previously published protocols [6,8,10]. Protocols such as those of D'Amour et al. [6,15] and Chetty et al. [7] using 100 ng/mL activin A generated eGFP+ cells at the highest percentage, consistent with the notion that high concentrations of activin A lead to high percentages of DE differentiation [26]. Our data also showed that increasing the activin A concentration from 50 to 100 ng/mL results in a high percentage of DE cells.
The GSK-3 inhibitor, CHIR99021, has been reported to support both DE and mesoderm differentiation from human PSCs through activation of Wnt signaling [20,27]. The specific cell fate is modulated by concentration and incubation time [27,28]. Upon high concentration and long incubation of CHIR99021, cells favor to develop toward the mesoderm; while upon low concentration short-term culture with an addition of activin A, cells favor to develop toward DE [27].
BMP4 represses cell pluripotency through BMP signaling [8,20]. With altered signaling time windows and interaction with different pathways, BMP4 has been reported to differentiate hESCs into various cell types. A long incubation of BMP4 will direct hESCs to the trophectoderm or primitive endoderm [29,30]. Maintaining NANOG expression by activation of activin and FGF signaling can promote BMP4-induced differentiation toward the mesendoderm instead of extraembryonic lineages [31]. A short-term incubation of BMP4 (no more than 24 h) will induce hESCs to generate mesendoderm/mesoderm progenitors [32]. Furthermore, combining BMP4 and activin A can promote DE formation [8].
BMP4 and CHIR99021 have similar effects in promoting DE formation [20]. However, no synergetic effect was found when applying both factors together with activin A. By combining BMP4 and CHIR99021 in Combined Protocol 4 and 7, the resulting eGFP+ percentage was ∼15% lower than using the two factors separately in Protocol 7 and Protocol 4, respectively. However, the total live cell number was the highest in Combined Protocol 4 and 7. As a result, the total eGFP+ cell numbers produced through the three modified protocols had no significant difference. The simultaneous activation of BMP and Wnt signaling does not promote further DE differentiation. We speculate that the reason for this might be due to the interaction between BMP and Wnt signaling. BMP signaling initiates Smad1 phosphorylation through activation of BMP receptors, mitogen-activated protein kinase, and GSK-3 [33,34]. Wnt signaling has a regulatory effect on the BMP/Smad1 signal [34]. Thus, the activation of both BMP and Wnt signaling may trigger the same downstream targets in promoting DE formation. However, it is unclear whether the DE differentiation potential of the two treatments (BMP4 vs. CHIR99021) would be different or not. In this study, we only differentiated DE further toward the pancreatic lineage, and DE derived from these two treatments shows no obvious differences.
A high concentration of FBS inhibits DE differentiation, so only a low concentration of FBS has been used in multiple DE differentiation protocols [15]. However, low serum in the first day of DE differentiation results in massive cell death, resulting in less than 10% of input cells left at the end of DE differentiation. By comparing multiple protocols, we found that B27 supplement can maintain a high cell survival rate on the first day of differentiation. This supplement was first developed to increase the survival of rat embryonic hippocampal neurons during in vitro culture [35], and it is beneficial for the culture of various cell types, including neural cells and stem cells [36,37]. We found here that with addition of 1% B27 during the first day of differentiation, the total live cell numbers increased significantly in the three modified protocols. Interestingly, prolonged B27 treatment from day 1 to 4 reduced eGFP+% (Protocol 8, Supplementary Table S1), suggesting that 1 day of B27 supplement is sufficient to improve DE differentiation.
This serum-free B27 contains 20 components, including vitamins, proteins, and other compounds [35]. Since B27 is a commercial product, in which the concentration of each component is confidential, we are not able to test each of the components. We tested four B27 substitutes: B27 without vitamin A (19 components of 20), B27 without antioxidants (15 components of 20), BSA fatty acid-free (one of the main component of B27), and N2 supplement (5 components of 20). Our results showed that BSA fatty acid-free alone can improve DE differentiation, although it is not as good as complete B27. BSA is widely used in serum-free culture of mammalian cells because albumin is a major protein in serum [38]. Albumin influences cell growth by mediating both the extracellular physical environment and intracellular biomolecular interactions [38]. Further investigation into the role of BSA and other components could help to reveal the molecular mechanisms of DE differentiation.
In vitro generation of insulin-producing beta cells derived from stem cells is attracting great attention in regenerative medicine. For example, Melton's group recently made a breakthrough in the production of functional beta cells [39]. Both hESCs and iPSCs were induced toward beta cells in a 35-day, six-stage in vitro suspension-based culture system. Activin A and CHIR99021 were applied in the DE induction stage and generated over 95% of SOX17+ cells. In spite of this high differentiation percentage, no cell survival rate was described. Because of the similarity of the Melton and Mod-D'Amour protocols, a high cell loss may have occurred. Since DE progenies generated through our modified protocols not only generated more endoderm cells but also more endoderm progenies, substituting the first stage of DE generation in Melton's protocol for our optimized DE protocols may lead to an increased number of live DE cells and a better overall yield of mature beta cells.
In summary, two criteria, total live cell number and percentage of eGFP+ cells, were used to optimize DE differentiation with the S17d5 reporter cell line. Three protocols (Protocol 7, Protocol 4, and Combined Protocol 4 and 7) were developed to improve DE differentiation. The use of 1% B27 supplement in these three protocols prevented massive cell death and greatly increased total DE cell number. Progenies of these optimized protocols could be further differentiated toward IPCs and lead to a significant increase of PDX1 and INS expression. These modified protocols also promoted DE differentiation in H1 hESCs and iPSCs. Therefore, the improved differentiation protocols for generating DE cells set a new basis for the next step of endoderm lineage differentiation.
Supplementary Material
Acknowledgments
The authors thank the members of the Wang group for materials, advice, and assistance. The authors also thank Jake Leighton for help in preparing the manuscript. Flow cytometry data were generated in the UTHSCSA Flow Cytometry Shared Resource Facility, which is supported by UTHSCSA, NIH-NCI P30 CA054174-20 (CTRC at UTHSCSA), and UL1 TR001120 (CTSA grant). The Wang group was supported by start-up funding from the Department of Cellular and Structural Biology, University of Texas Health Science Center at San Antonio. This work was also supported, in part, by grant DK047482.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Carpenter MK, Frey-Vasconcells J. and Rao MS. (2009). Developing safe therapies from human pluripotent stem cells. Nat Biotechnol 27:606–613 [DOI] [PubMed] [Google Scholar]
- 2.Keller G. (2005). Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev 19:1129–1155 [DOI] [PubMed] [Google Scholar]
- 3.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS. and Jones JM. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147 [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K. and Yamanaka S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872 [DOI] [PubMed] [Google Scholar]
- 5.Wang P, McKnight KD, Wong DJ, Rodriguez RT, Sugiyama T, Gu X, Ghodasara A, Qu K, Chang HY. and Kim SK. (2012). A molecular signature for purified definitive endoderm guides differentiation and isolation of endoderm from mouse and human embryonic stem cells. Stem Cells Dev 21:2273–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK. and Baetge EE. (2006). Production of pancreatic hormone–expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24:1392–1401 [DOI] [PubMed] [Google Scholar]
- 7.Chetty S, Pagliuca FW, Honore C, Kweudjeu A, Rezania A. and Melton DA. (2013). A simple tool to improve pluripotent stem cell differentiation. Nat Methods 10:553–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teo AKK, Ali Y, Wong KY, Chipperfield H, Sadasivam A, Poobalan Y, Tan EK, Wang ST, Abraham S, et al. (2012). Activin and BMP4 synergistically promote formation of definitive endoderm in human embryonic stem cells. Stem Cells 30:631–642 [DOI] [PubMed] [Google Scholar]
- 9.Tahamtani Y, Azarnia M, Farrokhi A, Sharifi-Zarchi A, Aghdami N. and Baharvand H. (2013). Treatment of human embryonic stem cells with different combinations of priming and inducing factors toward definitive endoderm. Stem Cells Dev 22:1419–1432 [DOI] [PubMed] [Google Scholar]
- 10.Pauklin S. and Vallier L. (2013). The cell-cycle state of stem cells determines cell fate propensity. Cell 155:135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannan NRF, Fordham RP, Syed YA, Moignard V, Berry A, Bautista R, Hanley NA, Jensen KB. and Vallier L. (2013). Generation of multipotent foregut stem cells from human pluripotent stem cells. Stem Cell Rep 1:293–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gage BK, Webber TD. and Kieffer TJ. (2013). Initial cell seeding density influences pancreatic endocrine development during in vitro differentiation of human embryonic stem cells. PLoS One 8:e82076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinner D, Rankin S, Lee M. and Zorn AM. (2004). Sox17 and β-catenin cooperate to regulate the transcription of endodermal genes. Development 131:3069–3080 [DOI] [PubMed] [Google Scholar]
- 14.Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PPL. and Hayashi Y. (2002). Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 129:2367–2379 [DOI] [PubMed] [Google Scholar]
- 15.D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E. and Baetge EE. (2005). Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 23:1534–1541 [DOI] [PubMed] [Google Scholar]
- 16.Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y. and Deng H. (2009). Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res 19:429–438 [DOI] [PubMed] [Google Scholar]
- 17.Wang P, Rodriguez RT, Wang J, Ghodasara A. and Kim SK. (2011). Targeting SOX17 in human embryonic stem cells creates unique strategies for isolating and analyzing developing endoderm. Cell Stem Cell 8:335–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madsen OD. and Serup P. (2006). Towards cell therapy for diabetes. Nat Biotechnol 24:1481–1483 [DOI] [PubMed] [Google Scholar]
- 19.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, et al. (2008). Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 26:443–452 [DOI] [PubMed] [Google Scholar]
- 20.Teo AKK, Valdez IA, Dirice E. and Kulkarni RN. (2014). Comparable generation of activin-induced definitive endoderm via additive Wnt or BMP signaling in absence of serum. Stem Cell Rep 3:5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon T-M, Chang B, Kim H-T, Jee J-H, Kim D-W. and Hwang D-Y. (2010). Human embryonic stem cells (hESCs) cultured under distinctive feeder-free culture conditions display global gene expression patterns similar to hESCs from feeder-dependent culture conditions. Stem Cell Rev Rep 6:425–437 [DOI] [PubMed] [Google Scholar]
- 22.Sepac A, Si-Tayeb K, Sedlic F, Barrett S, Canfield S, Duncan SA, Bosnjak ZJ. and Lough JW. (2012). Comparison of cardiomyogenic potential among human ESC and iPSC lines. Cell Transplant 21:2523–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin D, Tavakoli T, Gao W-Q. and Ma W. (2012). Comparison of neural differentiation potential of human pluripotent stem cell lines using a quantitative neural differentiation protocol. Methods Mol Biol 873:247–259 [DOI] [PubMed] [Google Scholar]
- 24.Mfopou JK, Geeraerts M, Dejene R, Van Langenhoven S, Aberkane A, Van Grunsven LA. and Bouwens L. (2014). Efficient definitive endoderm induction from mouse embryonic stem cell adherent cultures: a rapid screening model for differentiation studies. Stem Cell Res 12:166–177 [DOI] [PubMed] [Google Scholar]
- 25.McLean AB, D'Amour KA, Jones KL, Krishnamoorthy M, Kulik MJ, Reynolds DM, Sheppard AM, Liu H, Xu Y, Baetge EE. and Dalton S. (2007). Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells 25:29–38 [DOI] [PubMed] [Google Scholar]
- 26.Sulzbacher S, Schroeder IS, Truong TT. and Wobus AM. (2009). Activin A-induced differentiation of embryonic stem cells into endoderm and pancreatic progenitors-the influence of differentiation factors and culture conditions. Stem Cell Rev 5:159–173 [DOI] [PubMed] [Google Scholar]
- 27.Naujok O, Diekmann U. and Lenzen S. (2014). The generation of definitive endoderm from human embryonic stem cells is initially independent from Activin A but requires canonical Wnt-signaling. Stem Cell Rev Rep 10:480–493 [DOI] [PubMed] [Google Scholar]
- 28.Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG. and Little MH. (2014). Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol 16:118–126 [DOI] [PubMed] [Google Scholar]
- 29.Vallier L, Touboul T, Chng Z, Brimpari M, Hannan N, Millan E, Smithers LE, Trotter M, Rugg-Gunn P, Weber A. and Pedersen RA. (2009). Early cell fate decisions of human embryonic stem cells and mouse epiblast stem cells are controlled by the same signalling pathways. PLoS One 4:e6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu R-H, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP. and Thomson JA. (2002). BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol 20:1261–1264 [DOI] [PubMed] [Google Scholar]
- 31.Yu P, Pan G, Yu J. and Thomson JA. (2011). FGF2 sustains NANOG and switches the outcome of BMP4-induced human embryonic stem cell differentiation. Cell Stem Cell 8:326–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sui L, Bouwens L. and Mfopou JK. (2013). Signaling pathways during maintenance and definitive endoderm differentiation of embryonic stem cells. Int J Dev Biol 57:1–12 [DOI] [PubMed] [Google Scholar]
- 33.Sapkota G, Alarcón C, Spagnoli FM, Brivanlou AH. and Massagué J. (2007). Balancing BMP signaling through integrated inputs into the Smad1 linker. Mol Cell 25:441–454 [DOI] [PubMed] [Google Scholar]
- 34.Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM. and De Robertis EM. (2007). Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell 131:980–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brewer GJ, Torricelli JR, Evege EK. and Price PJ. (1993). Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res 35:567–576 [DOI] [PubMed] [Google Scholar]
- 36.Lamba DA, Karl MO, Ware CB. and Reh TA. (2006). Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A 103:12769–12774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seaberg RM. and van der Kooy D. (2002). Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci 22:1784–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francis GL. (2010). Albumin and mammalian cell culture: implications for biotechnology applications. Cytotechnology 62:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D. and Melton DA. (2014). Generation of functional human pancreatic β cells in vitro. Cell 159:428–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.