Abstract
Aims and background.
The circadian pattern of stroke occurrence variation has been recognized with certain differences between authors and stroke types. The underlying reason may be related to exogenous factors (cyclic physical activity, including sleep–awake cycles and assuming the up-right posture) and endogenous factors, with their diurnal variation (blood pressure, hemostatic balance, autonomic system activity). The aims of the present study are to investigate the existence of a circadian variation of stroke and the possible differences between stroke subtypes in the Cluj Napoca area.
Materials and method.
The stroke event data were acquired from the Patient Records of a consecutive series of 1083 patients admitted through the Emergency Room at Neurology Departments I and II of the District Hospital of Cluj Napoca, between 1 January 2012 and 31 December 2012. The classifiable onset time was assigned to one of four six-hour intervals: 00.01–06.00 (night), 06.01–12.00 (morning), 12.01–18.00 (afternoon) and 18.01–24.00 (evening). Demographic data and vascular risk factors were recorded.
Results.
All three stroke types (ischemic stroke, hemorrhagic stroke and subarachnoid hemorrhage) have shown a circadian variation regarding their occurrence, with the peak of incidence in the morning and the nadir during nighttime. This circadian pattern is independent by demographic factors and vascular risk factors.
Conclusion.
Our study confirmed the circadian variation of onset occurrence for all stroke subtypes. Some triggering factors promote ischemic stroke and prevent hemorrhagic stroke. The diurnal pattern of variation with the higher incidence in the morning and the lower in the night may lead to chrono-therapeutic and preventive approach (chrono-therapy of the risk factors), which targets the period of the highest vulnerability after awaking.
Keywords: stroke onset, circadian variation, stroke types
Aims and background
Stroke is the fourth leading cause of death and a major cause of adult disability. Relatively recent studies of survival in hypertensive patients have revealed that the incidence of stroke exceeded that of coronary events [1,2].
There are available data from observational studies suggesting that cerebrovascular events are not randomly distributed over time, but have a peculiar distribution along the day (circadian variation with morning peak of occurrence), week (circaseptan variation with higher frequencies in the last and first days of the week) and even months of the year (circannual or seasonal variation with predilection for the cold months) [3,4].
Various patterns of circadian onset variation have been reported for cerebrovascular events. Regarding the ischemic stroke (IS), the earliest studies suggested a higher incidence in evening hours or during nocturnal sleep [5]. Starting with the 1980s the studies more strongly indicate the morning higher occurrence of stroke with the lowest incidence during nighttime [6–9], confirmed by the conclusions of the meta-analysis of Elliot et al [10]. In the last years there were reports that showed a second peak, not so striking but evident, in the late afternoon [11–13]. There is an obvious similarity between cardiovascular events and cerebrovascular events regarding their diurnal variation with an increase during the morning hours after awaking and a decrease during the nocturnal sleep. This pattern of diurnal variation is typical for myocardial infarction, angina (stable, unstable, silent) and sudden death. This similarity suggests that cerebrovascular and cardiovascular events share common triggering factors [4,14,15].
For the hemorrhagic stroke (HS), the results of different studies show some differences, but the double-peak pattern is the most frequently reported [13,16–18]. The highest peak seems to be in the morning for some authors and [6,18] in the afternoon for others [10,11]. The most various patterns are reported regarding the subarachnoid hemorrhage (SAH), but all of them with the highest occurrence between 10.00 and 16.00 (with or without any peak) and the lowest during nighttime, suggesting the overlapping with the working hours [19,20].
Although the temporal pattern of stroke occurrence has been recognized, at least for the circadian variation of the stroke onset, the reason underlying the temporal nature of stroke is not completely understood. A constellation of exogenous cyclic factors such as temporal pattern in posture, physical activity, emotional stress and food behavior can influence the stroke occurrence. There are also data suggesting that endogenous factors, all having demonstrated a temporal variation, such as blood pressure (BP - with physiological nocturnal decrease and morning increase), autonomic system activity (with activation of the sympathetic nervous system after the wake-up moment) and hemostatic balance (with increased platelet aggregability, hypercoagulability and hypofibrinolysis in the morning), can influence the susceptibility to stroke or can even have a triggering role. Their physiological diurnal rhythms (related to the central and peripheral internal clock activity) and moreover, the alterations of this rhythm, are the most important factors contributing to the stroke occurrence, even bigger than the trigger itself [4,6,21,22].
The majority of available studies concerning the aspects of chronological variation refer to IS, there are less reports available for SAH and even HS, and only a few treating the three major types of stroke simultaneously.
The aims of our study are to investigate the existence of a circadian pattern in stroke occurrence, the relationship between this temporal pattern and the conventional vascular risk factors and the possible differences in the circadian variation among stroke types, in the Cluj Napoca area.
Materials and method
In the present retrospective, hospital–based study, we included a consecutive series of 1083 patients with stroke admitted through the Emergency Room to the Neurology Department I and II of the District Hospital of Cluj Napoca, between 1 January 2012 and 31 December 2012 (which serves the population of Cluj Napoca City and of the surroundings). The Cluj Napoca Metropolitan Area has a population of 411,379 inhabitants [23].
The study is focused simultaneously on the three major types of stroke: ischemic stroke (IS), stroke caused by intracerebral hemorrhage (HS) and stroke caused by subarachnoid hemorrhage (SAH) [24].
The diagnosis of IS was defined according to updated World Health Organization criteria, as an episode of neurological dysfunction caused by central nervous system (CNS: cerebral, spinal or retinal) infarction. The evidence of CNS infarction was defined as CNS cells death attributable to ischemia, based on imaging or other objective evidence of CNS focal ischemic injury in a defined vascular distribution, or on clinical evidence of CNS focal ischemia injury based on symptoms persisting ≥24 hours or until death. The diagnosis of HS was defined as rapidly developing clinical signs of neurological dysfunction attributable to a focal collection of blood within the brain parenchyma or ventricular system which is not caused by trauma. The diagnosis of SAH was defined as rapidly developing clinical signs of neurological dysfunction and/or headache caused by bleeding into the subarachnoid space, which is not secondary to a trauma [24].
For each patient demographic data (sex, age) were recorded, date of onset and the daytime period or hour, where it was specified, risk factors and comorbidities: arterial hypertension, diabetes mellitus, hypercholesterolemia, coronary artery disease (CAD), atrial fibrillation (AF) and smoking status. The lack of computed tomography within the first 48 hour was an exclusion criteria. Based on the data available in the Patient Records, precise or approximate determination of the time of symptoms onset was possible for 925 cases and was assigned to 1 of 4 six-hours intervals: 00.01–06.00 (night), 06.01–12.00 (morning), 12.01–18.00 (afternoon) and 18.01–24.00 (evening). For the remaining 158 patients the date of stroke onset could be determined; it was treated as a separate category in the descriptive analysis and was randomly redistributed along the daytime for Fourier curves analysis of circadian rhythm pattern. The wake-up strokes (the situation when the symptoms of stroke was first recognized to the wake-up moment) were assigned equally between 00.01–06.00 (night) and 06.01–12.00 (morning). According to the Patients Records, the number of wake-up stroke was 92 patients for IS (9.5%), 6 patients for IH (6.4%) and none for SAH.
The statistical analyses were performed using Excel Microsoft and SPSS software.
Results
The characteristics of the patients with stroke (having IS, SH or SAH) are shown in table I. Men represent 47.18% (572) and women represent 52.81% (511). The women’s average age is 72.26+/−11.09 years, compared with the men’s age 68.67+/−11.26 years.
Table I.
The demographic characteristics of the patients with stroke (IS, HS and SAH).
| Age | M mean (count) | F mean (count) |
|---|---|---|
| <45 y | 37.94 (16) | 41.91 (11) |
| 45–64 y | 58.34 (196) | 57.21 (107) |
| 65–80 y | 73.13 (279) | 73.58 (260) |
| >80 y | 84.4 (81) | 84.31 (133) |
|
| ||
| Total | 68.67 (572) | 72.26 (511) |
The total of 1083 patients were classified in the known three major types of stroke: 969 patients with IS (89.47%), 94 patients with HS (8.68%) and 20 patients with SAH (1.85%).
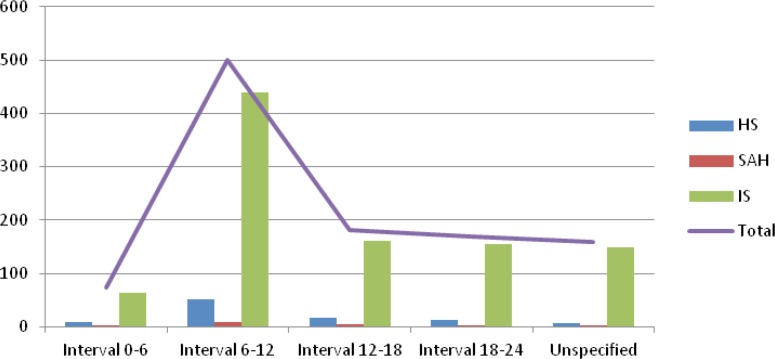
Figure 1 illustrates the distribution of all strokes and of each stroke type onset along the daytime concerning the four six-hours intervals convened. It shows an evident peak during the morning hour (corresponding to interval 06.01–12.00), followed by a slow decrease during afternoon (12.01–18.00), evening (18.01–24.00) and nighttime (interval 00.01–06.00) for each type of stroke. The percentage of occurrences during the four six-hour intervals for each type is described in table II.
Figure 1.
The distribution of IS, HS and SAH onset over the four six-hour intervals during the day.
Table II.
The percentage for each stroke type assigned to the four 6-hours intervals.
| Stroke type | Night (0–6) | Morning(6–12) | Afternoon(12–18) | Evening (18–24) | Undetermined |
|---|---|---|---|---|---|
| IS | 6.60% | 45.40% | 16.61% | 16.00% | 15.38% |
| HS | 8.51% | 55.31% | 17.02% | 12.76% | 6.38% |
| SAH | 10% | 40% | 20% | 15% | 15% |
IS – Ischemic Stroke, HS – Hemorrhagic stroke, SAH - Subarachnoid Hemorrhage.
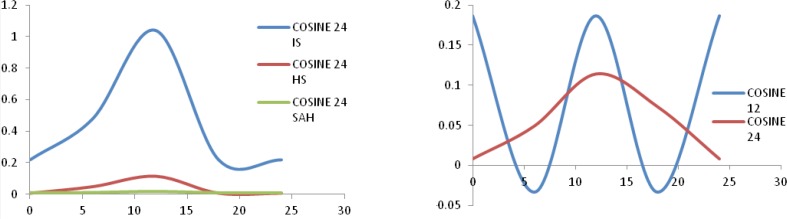
Using a Fourier analysis for the detection of the hypothesized circadian rhythm pattern (cosinor function), after the random distribution along the daytime of the patients with undetermined onset time, we found two cycles of 24 and 12 hours (Fig. 2). The cosinor indices for the 24 hours cycles are summarized in table III.
Figure 2.
The circadian variation using Fourier analysis (cosinor) for IS, HS and SAH (first box) and the two cycles of 12 hours and 24 hour for all strokes (second box).
Table III.
The parameters from Fourier analysis (cosinor) of 24 hours rhythms.
| Stroke Type | F | p | Mesor | Amplitude | Acrophase |
|---|---|---|---|---|---|
| IS | 101.306 | .000 | .63 | .43 | 13.3 |
| HS | 17.949 | .000 | .61 | .54 | 12.8 |
| SAH | 1.012 | .364 | .61 | 0.005 | 14.1 |
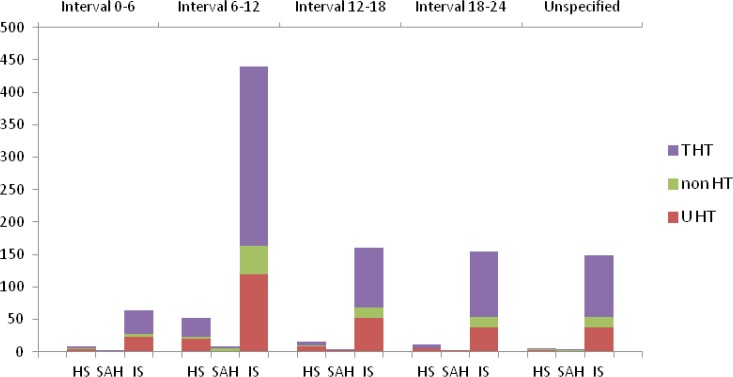
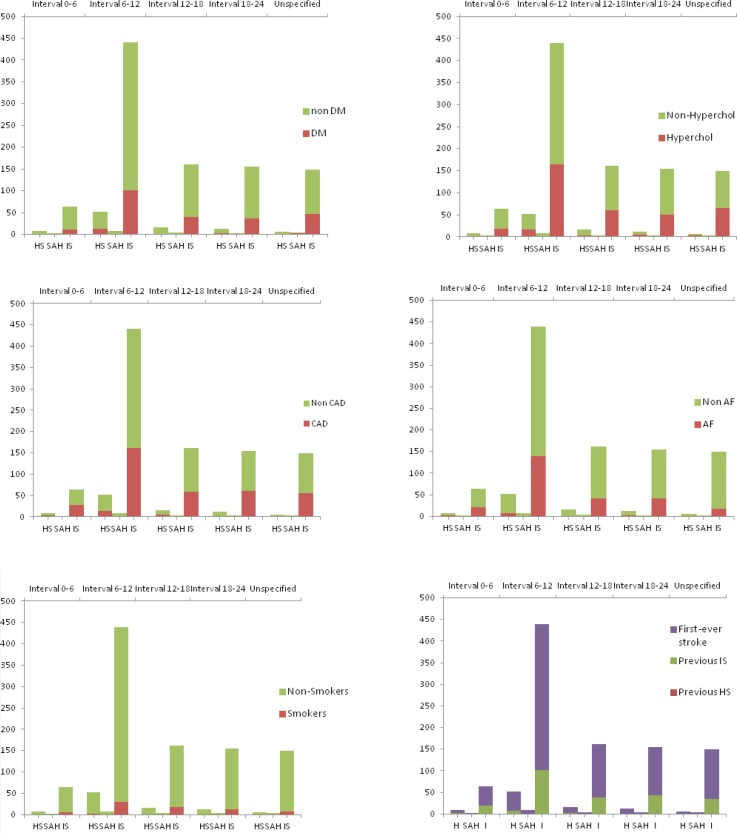
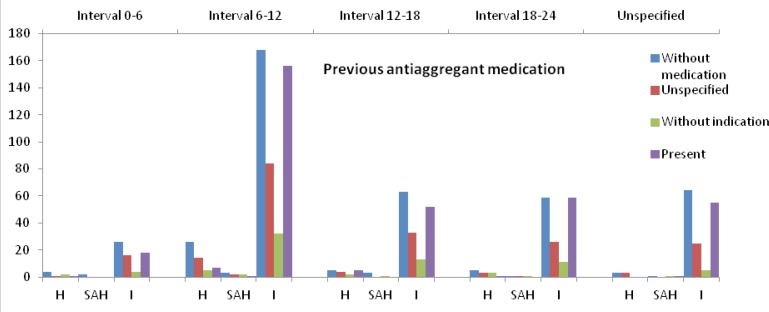
We analyzed the circadian variation in the condition of the presence/absence of the risk factors. The presence of the risk factors regarding each stroke type is described in table IV. The pattern of diurnal variation of stroke types concerning the risk factors: hypertension, diabetes mellitus, hypercholesterolemia, coronary artery disease, atrial fibrillation, smoking status and previous stroke are shown in the figures 3–4. The diurnal variation related to the previous anti-aggregant medication is shown in figure 5. The situation of the anticoagulant medication is similar with that of the anti-aggregant medication and is not mentioned in the article.
Table IV.
The risk factors evidenced in the patients with ischemic stroke.
| Risk factor | IS % | HS % | SAH % |
|---|---|---|---|
| Hypertension | 27.66% (268) | 42.55% (40) | 35%(7) |
| Diabetes Mellitus | 23.12%(224) | 15.96%(15) | 15%(3) |
| Hypercholesterolemia | 37.05% (359) | 53.19%(50) | 25% (5) |
| CAD | 37.67%(365) | 28.72(27) | 10%(2) |
| FiA | 26.93%(261) | 13.83%(13) | 5%(1) |
| Smoking habit | 7.32%(71) | 4.25%(4) | 5%(1) |
| Previous stroke | 24.4% (236) | 12.8% (12) | 0.5% (1) |
Figure 3.
The variation of stroke types concerning the absence/presence of treated/untreated arterial hypertension (non HT – non-hypertension, T HT- treated hypertension, U HT- untreated hypertension).
Figure 4.
The variation of stroke types related to the absence/presence of risk factors: DM (diabetes mellitus), hypercholesterolemia, coronary artery disease (CAD), atrial fibrillation (AF), smoking status and previous stroke.
Figure 5.
The circadian variation of stroke onset regarding the previous antiaggregant medication.
Discussion
The proportion between the three types of stroke found in our study is relatively close to that reported in literature [1]. Overall the men’s age is about 3.5 years lower than the age of women. The difference is more obvious in younger categories, becoming the same after 65 years (table I) and reflecting the onset or worsening of vascular risk factors after menopause [25].
The results of this study are in accordance with the findings of the majority of previous reports available in literature, with the highest incidence of stroke occurrence during the morning (6.01–12.00) and the minimum occurrence during the night (00.01–06.00) for all stroke types (IS, HS and SAH) (Fig. 1, table II) [6,18,26,27]. The spectral Fourier analysis of the time of stroke onset has shown the existence of two cycles: one of 12-hours and one of 24-hours. Analyzing the curves and indices of the two cycles (Fig. 2, table III) we can speculate that the cycles are phase-shifted by approximately 90 degrees. They suggest the possibility that initially the two cycles antagonize each other and after 12-hours they synchronize with a synergic effect. The 24 cosinor curve analysis shows a hall-mark of the 12-hours cycle in their first part. Therefore we can speculate the existence of a protective mechanism against stroke in the 00.01–06.00 interval. The nocturnal sleep seems “to protect” against stroke despite the old traditional teaching that strokes are more likely to occur at night [5,8], but further studies are needed to confirm this hypothesis, maybe including persons working on night shifts and changing their sleep cycle.
Some authors observed a single peak [6,7,9,13] in the morning, including Elliot’s meta-analysis, and others showing a double peak pattern of the circadian variation for all or for some of stroke types with a second small peak in the afternoon [11,26,28]. Their methodology included the division of the 24-hour daytime in more than four intervals, (six 4-hour, twelve 2-hour or even 24 1-hour), because the patients data from their Stroke Registries were more accurate and permitted an increased precision in the stroke onset time determination [7,9,11,18].
In our study, this pattern with significant increase in stroke incidence during morning–time was found even in the condition of an equal redistribution of the wake-up stroke number between the night and the morning interval, despite the consideration that the stroke concentration at the time of awakening was not due to the concentration of stroke occurrence, but to that of its recognition [8,11]. Concerning IS, in literature it was reported around 20%–25% of the wake-up stroke as compared to our findings (9.5%) [8,11].
The same circadian pattern for IS, HS and SAH suggests common triggers for all three types of stroke, the endogenous factors also having a known circadian variation. The best studied triggers are the blood pressure (BP) and the coagulation status. The BP has a well-known circadian variation with a nocturnal dip and a morning increase. The loss of the physiological circadian pattern of BP (non-dipper and reverse-dipper profile) and the excessive morning BP surge at wake-up are accompanied by an increased risk of cerebrovascular events. Both aspects are characterized by high morning BP [29–31]. We obtained information only for the previous hypertension status, and in our findings the circadian pattern of stroke onset was almost the same in hypertensive and non-hypertensive patients with or without antihypertensive treatment (Fig. 3). The same situation is suggested for the other vascular risk factors (DM, hypercholesterolemia, smoking status, AF and CAD) with the same circadian variation in their presence or absence (Fig. 4). During the morning hours, physiologically the hemostatic balance is inclined to a temporal prothrombotic status, with increased levels of the hematocrit, increased platelet aggregability, hypercoagulability and hypofibrinolytic activity. We do not have information about the hemostatic status before the stroke and we could not analyze this aspect, but we recorded the situation of previous prophylactic anti-aggregant and anticoagulant medication, which did not modify the circadian pattern of stroke occurrence (Fig. 5).
The two best known triggering factors, high morning BP and pro-thrombotic status in the morning, promote ischemic stroke and the latter prevents hemorrhagic stroke (a conclusion of the studies that found the highest peak for HS and SAH in the afternoon) [11]. Our study found one peak for HS in the morning with a relatively increased level of occurrence in the afternoon (12.01–18.00) and evening (18.01–24.00) as compared to night. SAH seems to have a similar situation but the number of SAH cases was too small for a strong conclusion, reports in literature relating SAH occurrence time to the working period of the day [32].
There are reports suggesting the modification of BP circadian rhythm post stroke (with reduction of nocturnal BP decline) the but we did not find a more impressive peak in the morning for the patients with previous stroke in comparison with the patients with the first-ever stroke (Fig. 4) [8].
The highest occurrence of stroke during morning hours has a sociological dimension as information, with practical implication for the admission, evaluation and treatment of stroke in Emergencies Departments/Stroke Units, requiring an increased level of awareness and availability during the morning interval. Another wide implication may be the prevention of stroke by treating the risk factors concerning their circadian variation. A chrono-therapeutic approach may be useful for arterial hypertension (started to develop in the last years) [33], in the case of anticoagulant treatment [34] and possible in the case of antiplatelet treatment [35,36]. In order to address this problem further studies with long-term investigations are needed.
Conclusions
To our knowledge, this is the first Romanian study concerning the circadian variation of stroke onset. Our results show that all three type of stroke have a circadian variation with the highest peak in the morning and the lowest during nighttime. This pattern of variation is present independently of the presence of vascular risk factors (arterial hypertension, DM, hypercholesterolemia, CAD, AF and smoking status), of previous stroke and demographic aspects. The triggering factors are common, the most important and studied being the diurnal rhythm of blood pressure and the hemostatic balance. These findings may have therapeutic applicability (regarding the acute intervention treatment like thrombolysis) and implications for prophylaxis (chrono-therapy of the risk factors).
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kjeldsen SE, Julius S, Hedner T, Hansson L. Stroke is more common than myocardial infarction in hypertension: analysis based on 11 major randomized intervention trials. Blood Press. 2001;10(4):190–192. doi: 10.1080/08037050152669684. [DOI] [PubMed] [Google Scholar]
- 3.Manfredini R, Gallerani M, Portaluppi F, Salmi R, Fersini C. Chronobiological patterns of onset of acute cerebrovascular diseases. Thromb Res. 1997;88(6):451–463. doi: 10.1016/s0049-3848(97)00286-7. [DOI] [PubMed] [Google Scholar]
- 4.Schallner N, LeBlanc R, Otterbein LE, Hanafy KA. Circadian Rhythm in Stroke – The Influence of Our Internal Cellular Clock on Cerebrovascular Events. J Clin Exp Pathol. 2014;2014;4:163. [Google Scholar]
- 5.Hossmann V, Zulch KJ. Circadian Variations of Hemodynamics and Stroke. Brain and Heart Infarct II. 1979;II:171–180. [Google Scholar]
- 6.Manfredini R, Boari B, Smolensky MH, Salmi R, la Cecilia O, Maria Malagoni A, et al. Circadian variation in stroke onset: identical temporal pattern in ischemic and hemorrhagic events. Chronobiol Int. 2005;22(3):417–53. doi: 10.1081/CBI-200062927. [DOI] [PubMed] [Google Scholar]
- 7.Casetta I, Granieri E, Fallica E, la Cecilia O, Paolino E, Manfredini R. Patient demographic and clinical features and circadian variation in onset of ischemic stroke. Arch Neurol. 2002;59(1):48–53. doi: 10.1001/archneur.59.1.48. [DOI] [PubMed] [Google Scholar]
- 8.Lago A, Geffner D, Tembl J, Landete L, Valero C, Baquero M. Circadian variation in acute ischemic stroke: a hospital-based study. Stroke. 1998;29(9):1873–1875. doi: 10.1161/01.str.29.9.1873. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Shetty H. Circadian variation in stroke - a prospective hospital-based study. Int J Clin Pract. 2005;59(11):1272–1275. doi: 10.1111/j.1368-5031.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- 10.Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke. 1998;29(5):992–996. doi: 10.1161/01.str.29.5.992. [DOI] [PubMed] [Google Scholar]
- 11.Omama S, Yoshida Y, Ogawa A, Onoda T, Okayama A. Differences in circadian variation of cerebral infarction, intracerebral haemorrhage and subarachnoid haemorrhage by situation at onset. J Neurol Neurosurg Psychiatry. 2006;77(12):1345–1349. doi: 10.1136/jnnp.2006.090373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spengos K, Vemmos KN, Tsivgoulis G, Synetos A, Zakopoulos NA, Zis V, et al. Two-peak temporal distribution of stroke onset in Greek patients. a hospital-based study. Cerebrovasc Dis. 2003;15(1–2):70–77. doi: 10.1159/000067129. [DOI] [PubMed] [Google Scholar]
- 13.Butt MU, Zakaria M, Hussain HM. Circadian pattern of onset of ischaemic and haemorrhagic strokes, and their relation to sleep/wake cycle. J Pak Med Assoc. 2009;59(3):129–132. [PubMed] [Google Scholar]
- 14.Stergiou G. Chronobiology and Stroke. Hellenic J Cardiol. 2004;45:242–245. [Google Scholar]
- 15.Shaw E, Tofler GH. Circadian rhythm and cardiovascular disease. Curr Atheroscler Rep. 2009;11(4):289–295. doi: 10.1007/s11883-009-0044-4. [DOI] [PubMed] [Google Scholar]
- 16.Feng J, Zhang JH, Qin X. Timing pattern of onset in hypertensive intracerebral hemorrhage patients. Acta Neurochir Suppl. 2011;111:327–331. doi: 10.1007/978-3-7091-0693-8_54. [DOI] [PubMed] [Google Scholar]
- 17.Stergiou GS, Vemmos KN, Pliarchopoulou KM, Synetos AG, Roussias LG, Mountokalakis TD. Parallel morning and evening surge in stroke onset, blood pressure, and physical activity. Stroke. 2002;33(6):1480–1486. doi: 10.1161/01.str.0000016971.48972.14. [DOI] [PubMed] [Google Scholar]
- 18.Turin TC, Kita Y, Rumana N, Nakamura Y, Takashima N, Ichikawa M, et al. Is there any circadian variation consequence on acute case fatality of stroke? Takashima Stroke Registry, Japan (1990–2003) Acta Neurologica Scandinavica. 2012;125(3):206–212. doi: 10.1111/j.1600-0404.2011.01522.x. [DOI] [PubMed] [Google Scholar]
- 19.Temes RE, Bleck T, Dugar S, Ouyang B, Mohammad Y, John S, et al. Circadian variation in ictus of aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2012;16(2):219–223. doi: 10.1007/s12028-011-9640-6. [DOI] [PubMed] [Google Scholar]
- 20.Feigin VL, Anderson CS, Rodgers A, Bennett DA. Subarachnoid haemorrhage occurrence exhibits a temporal pattern - evidence from meta-analysis. Eur J Neurol. 2002;9(5):511–516. doi: 10.1046/j.1468-1331.2002.00455.x. [DOI] [PubMed] [Google Scholar]
- 21.Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension. 2010;56(5):765–773. doi: 10.1161/HYPERTENSIONAHA.110.157149. [DOI] [PubMed] [Google Scholar]
- 22.Ali K, Leong KM, Houlder S, Getov S, Lee R, Rajkumar C. The relationship between dipping profile in blood pressure and neurologic deficit in early acute ischemic stroke. J Stroke Cerebrovasc Dis. 2011;20(1):10–15. doi: 10.1016/j.jstrokecerebrovasdis.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Rezultate definitive ale Recensamantului Populatiei si Locuintelor - 2011 - analiza. Cluj County Regional Statistics Directorate; Epub 2013-07-05. [Google Scholar]
- 24.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st Century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bushnell CD. Stroke and the female brain. Nat Clin Pract Neurol. 2008;4(1):22–33. doi: 10.1038/ncpneuro0686. [DOI] [PubMed] [Google Scholar]
- 26.Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology. 2013;81(3):264–272. doi: 10.1212/WNL.0b013e31829bfde3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wouters A, Lemmens R, Dupont P, Thijs V. Wake-up stroke and stroke of unknown onset: a critical review. Front Neurol. 2014;5:153. doi: 10.3389/fneur.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turin TC, Kita Y, Rumana N, Takashima N, Ichikawa M, Sugihara H, et al. Morning surge in circadian periodicity of ischaemic stroke is independent of conventional risk factor status: findings from the Takashima Stroke Registry 1990–2003. Eur J Neurol. 2009;16(7):843–851. doi: 10.1111/j.1468-1331.2009.02605.x. [DOI] [PubMed] [Google Scholar]
- 29.Gosse P, Schumacher H. Early morning blood pressure surge. J Clin Hypertens (Greenwich) 2006;8(8):584–589. doi: 10.1111/j.1524-6175.2006.04773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel PV, Wong JL, Arora R. The morning blood pressure surge: therapeutic implications. J Clin Hypertens (Greenwich) 2008;10(2):140–145. doi: 10.1111/j.1751-7176.2008.07427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kario K. Time for focus on morning hypertension: pitfall of current antihypertensive medication. Am J Hypertens. 2005;18(2 Pt 1):149–151. doi: 10.1016/j.amjhyper.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Gallerani M, Manfredini R, Ricci L, Cocurullo A, Goldoni C, Bigoni M, et al. Chronobiological aspects of acute cerebrovascular diseases. Acta Neurologica Scandinavica. 1993;87(6):482–487. doi: 10.1111/j.1600-0404.1993.tb04141.x. [DOI] [PubMed] [Google Scholar]
- 33.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27(8):1629–1651. doi: 10.3109/07420528.2010.510230. [DOI] [PubMed] [Google Scholar]
- 34.Haus E. Chronobiology of hemostasis and inferences for the chronotherapy of coagulation disorders and thrombosis prevention. Adv Drug Deliv Rev. 2007;59(9–10):966–984. doi: 10.1016/j.addr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Bonten TN, Saris A, van Oostrom MJ, Snoep JD, Rosendaal FR, Zwaginga J, et al. Effect of aspirin intake at bedtime versus on awakening on circadian rhythm of platelet reactivity. A randomised cross-over trial. Thromb Haemost. 2014;112(6) doi: 10.1160/TH14-05-0453. [DOI] [PubMed] [Google Scholar]
- 36.Kriszbacher I, Koppan M, Bodis J, Yip HK, Chen SS, Chen MC. Aspirin for Stroke Prevention Taken in the Evening? Stroke. 2004;35(12):2760–2762. doi: 10.1161/01.STR.0000147155.08852.12. [DOI] [PubMed] [Google Scholar]