Abstract
This study aims to introduce an alternative technique for effective single-site robotic cholecystectomy (SSRC) using a reverse port.
Proper exposure of Calot's triangle is critical for safe laparoscopic cholecystectomy. Current robotic surgical systems are useful for single-site cholecystectomy. However, in exposing Calot's triangle, the gallbladder is usually retracted in a medial and upward direction, resulting in a narrow triangle. This intraoperative view is a major obstacle to safe laparoscopic cholecystectomy.
From October 2013 to October 2014, 55 consecutive patients underwent SSRC by a single surgeon at Yonsei University Severance Hospital. Initially, 5 patients underwent the original robotic single site cholecystectomy technique, and the remaining 50 patients underwent robotic single site cholecystectomy using our reverse port technique.
There were no differences between the SSRC-O (original port) group and the SSRC-R (reverse port) group in terms of patient age (P = 0.244), body mass index (P = 0.503), and pathologic conditions of the gallbladder (P = 0.841). Total operation time (132.6 vs 99.12 min; P = 0.009), actual dissection time (51.6 vs 30.28 min; P = 0.001), and console time (84.4 vs 50.46 min; P = 0.001) were all significantly shorter in the SSRC-R group. Mean intraoperative blood loss was minimal in both groups (20 vs 12.4 mL, P = 0.467), and bile spillage occurred in 2 patients of the SSRC-R group. There was one case of laparoscopic conversion in the SSRC-R group.
The reverse port technique described in this study successfully widened Calot's triangle and improved the safety of the current robotic surgical system for single-site robotic cholecystectomy.
INTRODUCTION
Laparoscopic cholecystectomy (LC) has been a gold standard treatment modality for benign pathologic conditions of the gallbladder from the early 1990s.1–3 With the accumulation of experience and advances in instrument design, LC has become a safer and more familiar procedure. Although conventional 4-port LC is the standard method, there has been a great effort to reduce the number and size of ports in order to achieve the goal of performing truly minimally invasive surgery. Recently single-incision laparoscopic cholecystectomy (SILC) has been performed by many surgeons.4–9 However, despite these novel advances in SILC, technical and ergonomic problems remained, such as instrument crowding, ergonomic discomfort experienced by the operator, difficulties in gallbladder retraction, and poor exposure of Calot's triangle.4,10 Due to these limitations, SILC can be performed by only a small number of experienced surgeons and long learning curve time is required,11 which has prevented the frequent application of SILC in benign gallbladder disease.
In 2011, the Da Vinci Surgical system (Intuitive surgical Inc, Sunnyvale, CA) developed a set of single-site instruments and accessories (R-LESS, robotic laparo-endoscopic single-site surgery).12 After the development of R-LESS, several surgeons reported their early experiences of single-site robotic cholecystectomy (SSRC).13–21 We also began performing SSRC using R-LESS from October 2013; however, we felt that dissection of Calot's triangle was uncomfortable and not safe mainly due to the narrow triangle that resulted from the medial upward retraction of the gallbladder caused by the assistant port.22 One of the most important points for “safe” cholecystectomy is ensuring full exposure of Calot's triangle by retracting the gallbladder in a cephalad and right lateral direction. This traction is intended to facilitate the opening Calot's triangle, leading to a widening angle between the cystic duct and common bile duct. This widened angle allows the surgeon to perform selective dissection of the cystic duct and cystic artery away from the common bile duct, thereby avoiding unnecessary bile duct injury.23–25 However, we found that the right lateral retraction of the gallbladder was very difficult during SSRC using the commercialized da Vinci single site surgical port, as the assistant port access was located at the left side of the camera port access (Fig. 1A–C).
FIGURE 1.
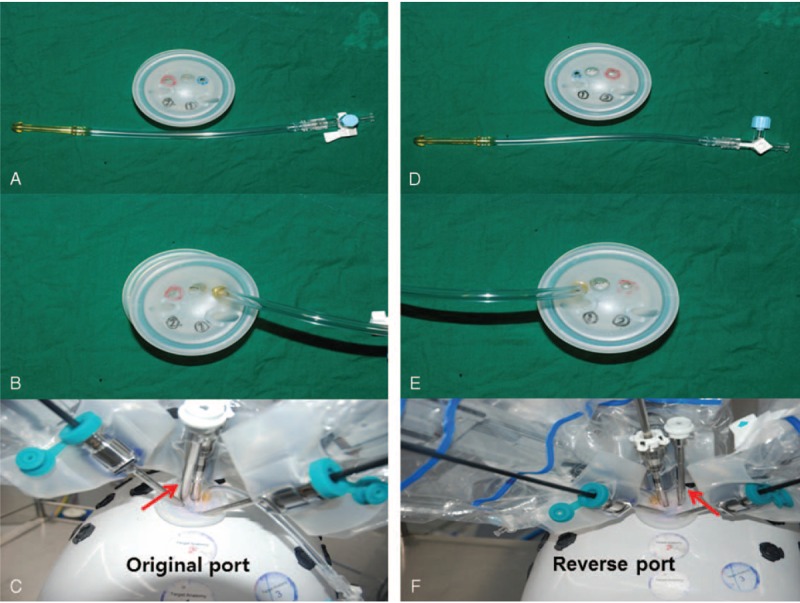
Original Da-Vinci single port platform: (A) red and blue circles indicate assistant port access and CO2 insufflation access, respectively. (B) CO2 insufflation line insertion status. (C) Red arrow indicates the assistant port. Assistant port is located at the left side of the camera port. Reverse single port platform: (D) red and blue circles indicate assistant port access and CO2 insufflation access, respectively. (E) CO2 insufflation line insertion status. (F) Red arrow indicates the assistant port. Assistant port is located at the right side of the camera port.
In this dynamic manuscript, we described a reverse port technique for SSRC. For the effective gallbladder retraction, we turned the port platform upside down to relocate the assistant port site to the right side of camera port. We found reverse port technique to be very useful and more feasible for performing safe SSRC than the original technique.
MATERIALS AND METHODS
Patients
This study was a retrospective review that was approved by our institutional review board. From October 2013 to October 2014, 55 consecutive patients underwent SSRC by a single surgeon at Yonsei University Severance Hospital. Initially, 5 consecutive patients underwent SSRC using the original da Vinci single port and the remaining 50 patients underwent SSRC using our reverse port technique. Indocyanin green (ICG; 2.5 mg) was administered via intravenous access to all patients ∼40 min before operation in order to provide intraoperative real-time fluorescent cholangiography.
All patients with benign gallbladder disease (ie, cholelithiasis, polyps, adenomyomatosis) were considered to for SSRC. Among them we excluded those with definite gallbladder empyema or severe acute cholecystitis who needed emergency cholecystectomy or preoperative percutaneous transhepatic gallbladder drainage on preoperative image study. A history of previous abdominal surgery and severe obesity were not absolute contraindications; nevertheless, they were considered as unsuitable cases.
Patients’ demographics collected included (sex, age, symptoms, history, BMI and diagnosis, and ASA score) and perioperative outcomes included (operation time, console time, blood loss, bile spillage during operation, conversion to open or laparoscopic surgery). Operation time was defined as the full operation time, including the docking time, actual dissection time, and console time. Actual dissection time was defined as the time from the beginning of the dissection of Calot's triangle to the end of the gallbladder removal from the liver bed. We analyzed general characteristics and intraoperative features between the original single port (SSRC-O) group and the reverse single port (SSRC-R) group.
Preparation of “reverse” port from original da Vinci port—Supplementary Video clip 1,http://links.lww.com/MD/A474
Remove the previously inserted CO2 insufflation catheter gently.
Turn the port upside down.
Reinsert the removed insufflation catheter through the port. A small amount of surgical gel may be used to facilitate this procedure. Then you can see the assistant port access is located on the right side of camera port access (Fig. 1D–F).
Operative procedure (reverse port method)—Supplementary Video clip 2,http://links.lww.com/MD/A475
The entire procedure was similar to the previously reported technique. In brief, each patient was placed in the supine position on the operation table and underwent general endotracheal anesthesia. The abdomen was draped with a usual sterile manner. A 2.5- to 3-cm transumbilical vertical skin incision was made through the midpoint of the umbilicus. The fascia and peritoneum were opened in the longitudinal direction using the Hassan technique; the incision was enlarged to 2.5 cm. The reversed port, which had already been prepared, was inserted into the abdomen, and CO2 gas was infused via the insufflations port access to create a pneumoperitoenum up to a pressure of 12 to 15 mm Hg. The table was placed in a partial reverse Trendelenburg position (15–20°) and rotated on the right axis by 30° to allow the da Vinci robot arms to be placed on the table. Initially, an 8.5-mm endoscope was introduced through the camera port, and with the guidance of this endoscope, 2 curved 5-mm cannulas were introduced via the port access and placed at the appropriate position (near the gallbladder). After the docking was completed, a 5-mm assistant cannula was introduced through the accessory port access, which was located on the right side of camera port access in the reverse port.
The operator then moved to the surgical console, and the assistant surgeon took position on the left side of the patient—an exact mirror image of the conventional technique (Fig. 2). At the beginning of the operation, the assistant held the fundus of the gallbladder using a long straight grasper forceps via the assistant port access. Then, the operator changed the position of endoscope to below the assistant grasper forceps and advanced, resulting in the retraction the gallbladder in a cephalad and right lateral direction. This movement was able to solve the problem of crowding between the endoscope and assistant grasper forceps, and Calot's triangle was opened widely, in contrast to the conventional technique, producing an optimal environment for safe cholecystectomy (Fig. 2). The operator performed the cholecystectomy using several da Vinci robot instruments, including the Maryland dissector, monopolar cautery, crocodile grasper, scissors, and Hem-o-lok clip. During the operation the operator checked the anatomy of the biliary tree (common bile duct and cystic duct) using intraoperative infrared fluorescent cholangiography. After the gallbladder was detached from the liver, the gallbladder was removed via the umbilical incision with the 5-mm specimen bag. The fascia defect was closed with continuous reabsorbable suture and the skin was approximated with subcuticular continuous suture.
FIGURE 2.
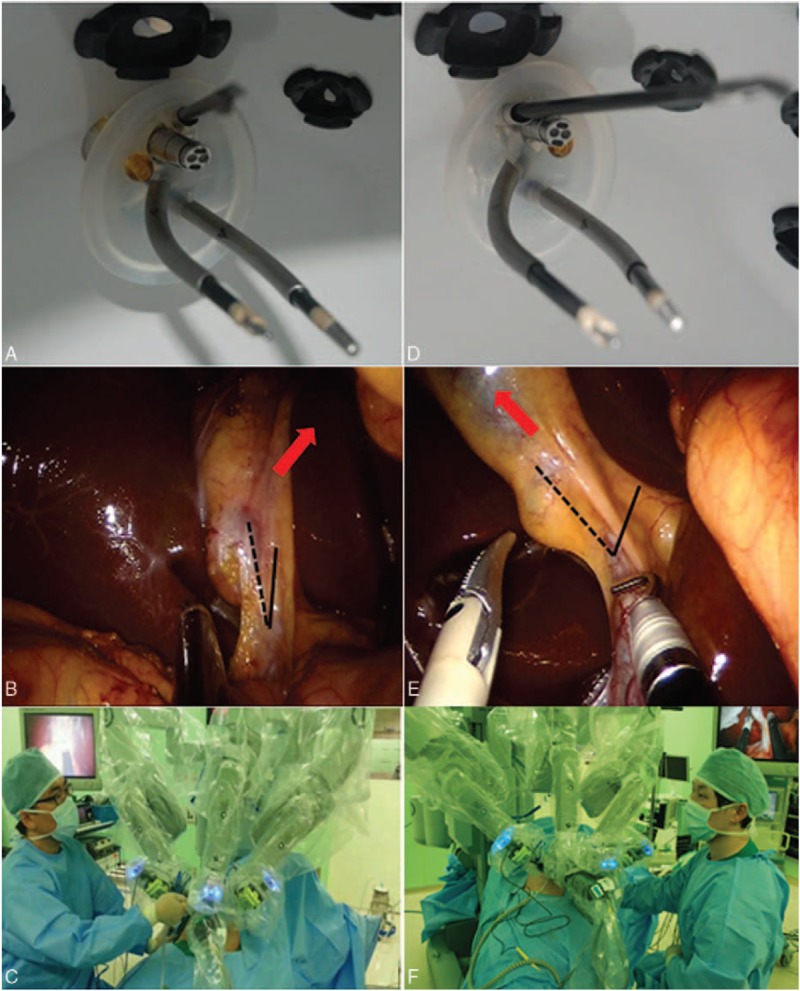
Internal view of the direction of the assistant port using (A) the original port and (D) the reverse port. (B) Exposure of Calot's triangle using the original port; red arrow indicates the direction of gallbladder traction by the assistant port. The angle between CBD (solid line) and cystic duct (dotted line) is narrow. (E) Exposure of Calot's triangle using the reverse port. The direction of gallbladder traction is toward the right lateral direction (red arrow); the angle between CBD and cystic duct is more widened. The position of the assistant during operation: (C) SSRC using the original port, assistant positioned at the right side of the patient. (F) SSRC using the reverse port, assistant positioned at the left side of the patient.
Statistical Analysis
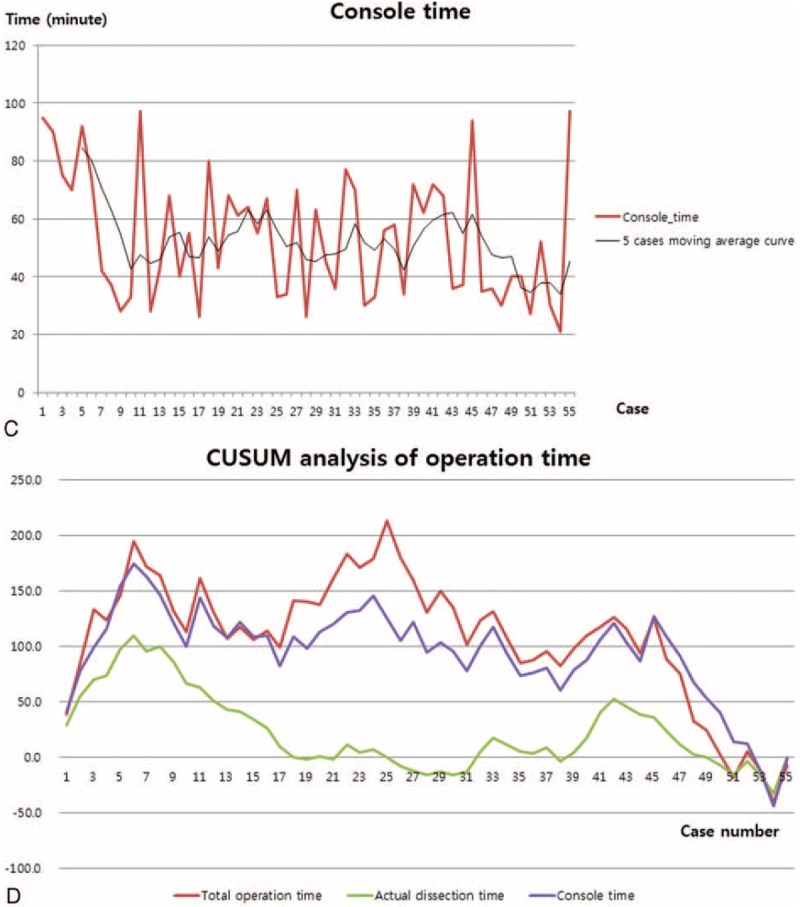
Categorical variables are expressed as the number and percentage, and continuous variables are expressed as mean values ± standard deviation. The chi-square test was used for categorical variables, and the Mann–Whitney U test was used for continuous variables to analyze statistical significance between parameters. We used the cumulative sum control chart (CUSUM) technique and the moving average curve to analyze the quantitative assessment of the learning curve. A typical learning curve created using the CUSUM technique shows an initial upward slope, indicating a period of acquiring experience. After this upward slope, a downward slope or a plateau follows, indicating achievement of the learning curve at that moment. A P value <0.05 was regarded as indicating statistical significance.
RESULTS
General characteristics and intraoperative outcomes of all patients are shown in Table 1. All patients were diagnosed with a benign gallbladder disease. There were no differences in age (P = 0.244), BMI (P = 0.503), pathologic diagnosis (P = 0.841), or ASA score (P = 0.744) between the 2 groups (Table 1). There was only one case of laparoscopic conversion in the SSRC-R group. On comparative analysis between the SSRC-O group and the SSRC-R group, it was observed that total operation time (132.6 vs 99.12 min, P = 0.009), actual dissection time (51.6 vs 30.28 min, P = 0.001), and console time (84.4 vs 50.46 min, P = 0.001) were significantly shorter in the SSRC-R group. Estimated blood loss was minimal, and mild bile spillage during the dissection of the gallbladder from the liver bed occurred in 2 patients in the SSRC-R group (Table 2). All patients were discharged within 2 days after operation without complications. Operative time, particularly actual dissection time, was shown to be dramatically shorter after the introduction of the reverse port technique. On CUSUM analysis, the learning curves of the total operation time and console time were reached at ∼40 cases, whereas the learning curve of the actual dissection time was reached at ∼20 cases (Fig. 3 ).
TABLE 1.
General Characteristics of Patients Who Underwent Single Site Robotic Cholecystectomy Using the Original Port and the Reverse Port
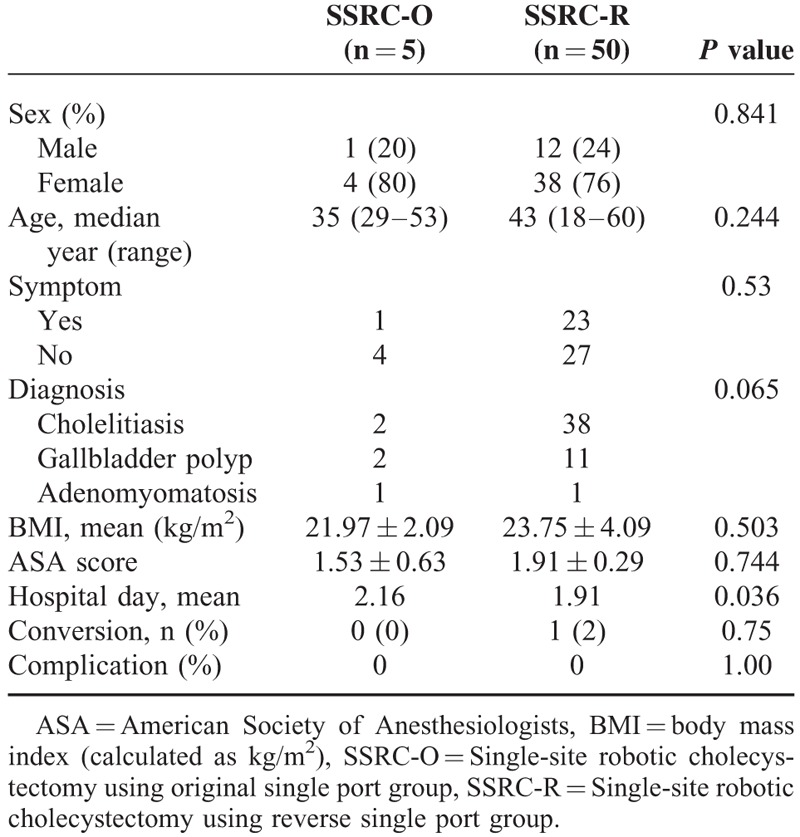
TABLE 2.
Comparison of Perioperative Outcomes Between the Original Single Port and the Reverse Single Port Group
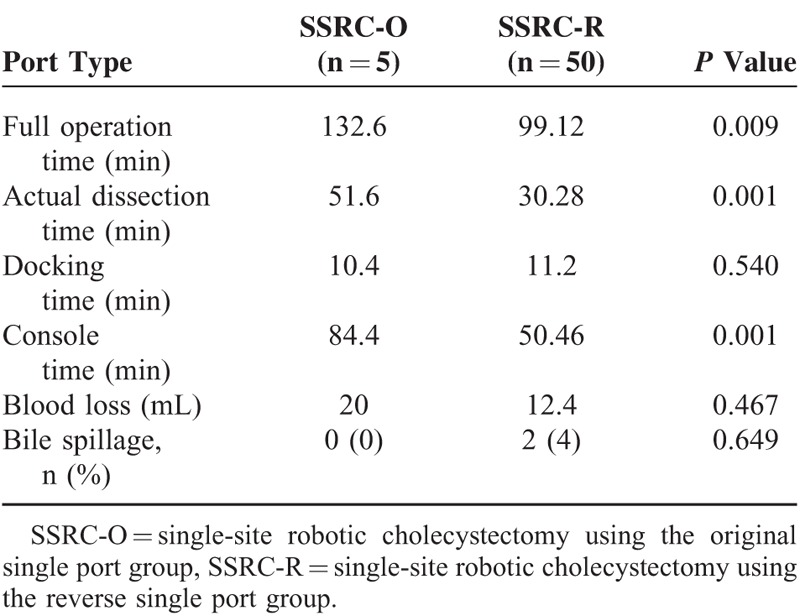
FIGURE 3.
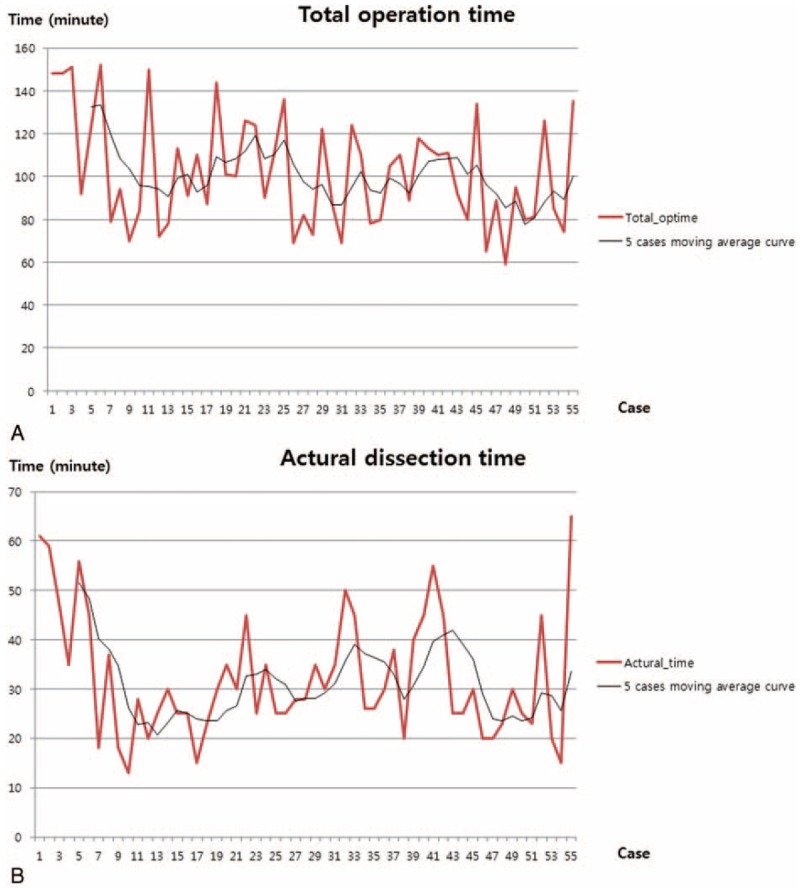
Operation times for the sequence of 55 patients. (A–C) demonstrate each moving average time of total operation time, actual dissection time, and console time, respectively. (D) shows CUSUM slope of each operation time, after the first 5 cases (the starting point of the use of reverse port); every slope showed abrupt declining curves. CUSUM = cumulative sum control chart.
FIGURE 3 (Continued).
Operation times for the sequence of 55 patients. (A–C) demonstrate each moving average time of total operation time, actual dissection time, and console time, respectively. (D) shows CUSUM slope of each operation time, after the first 5 cases (the starting point of the use of reverse port); every slope showed abrupt declining curves. CUSUM = cumulative sum control chart.
DISCUSSION
In 2011, da Vinci robot intuitive introduced R-LESS.12 Cholecystectomy was introduced for single-site surgical procedure. Several institutions began performing SSRC with this novel platform and reported the outcomes of their early experiences. Previous articles focused on the advantages of SSRC compared to SILC,13–18,20,21 and we also agreed with these proposed potential advantages. However, the current R-LESS system still have some technical problem to be resolved for safe and effective cholecystectomy. These limitations may consequently prevent the widening of indications for SSRC over SILC.
During the initial experience of SSRC using the original technique, we felt that gallbladder retraction toward the right-lateral direction was very difficult with the conventional R-LESS system. This situation can result in the potential risk of unnecessary injury to the bile duct and vascular structure during cholecystectomy, as the anatomic landmark of Calot cannot be widely opened. Thus, we used a reverse port to solve this small yet critical technical problem. Our technique was very easy and did not require additional costs. With this simple manipulation, the surgeon was able to obtain an optimal operative view with the widened Calot's triangle and stick to the principle of safe cholecystectomy during SSRC. With these advantages, we were able to expand our indications for SSRC. Particularly, patients presenting with a narrow Calot's triangle and a short cystic duct (frequently caused by acute gallbladder inflammation and obesity) were found to greatly benefit from the reverse port technique.
We acknowledge that the number of patients in the original port group was too small to perform a reliable statistical comparison; however, we found that the operation view was preferable and stable, and the procedure itself was very effective. As a result, the actual dissection time was dramatically reduced to ∼20 min (Fig. 3 ), and we were also able to shorten the learning-curve period. The reason why we used the actual dissection time in the analysis was because there were several adverse biases relating to the total operation time and console time, particularly that the education of residents and fellows was time consuming.
In addition, intraoperative infrared fluorescent cholangiography was very helpful preventing bile duct injury and indicated the proper dissection plane during the SSRC. As already reported in prior articles, this simple procedure can be greatly beneficial during SSRC.26–28
Our study had several limitations that necessitated further studies to verify the role of the reverse port technique as a routine clinical practice.
First, this was a retrospective designed study with a small number of samples comprising our early experiences. Therefore, it was expected to harbor inevitable selection bias.
Second, as mentioned briefly above, the original port group comprised only 5 patients, in contrast to the 50 patients of reverse port group. As such, reliable statistical results to proving the superiority of the reverse port technique could not be obtained. However, based on our current observation, which indicated a dramatic shortening of the actual dissection time, a future randomized control study may validate our result.
Finally, although the current R-LESS platform has overcome several of the limitations of SILC, more advance are required to extend the spectrum of SSRC. The technical requirements to improve SSRC are as follows:
More articulated instruments will increase the degree of movement and eventually enable more complex operations.
Due to the lack of articulating movement in the effector instrument, a more angulated dissector than the Maryland dissector, such as right-angled dissector, may be helpful during the dissection of Calot's triangle.
Occasionally, Hem-o-lok clips seemed to be too large to be applied in the narrow space of Calot's triangle. A small, straight clip system will be very effective in such cases.
In conclusion, SSRC using the reverse port technique can be used as an alternative for effective single-site robotic cholecystectomy. It was shown to provide an excellent operative field and a wider Calot's triangle. We expect SSRC using our reverse port technique to be able to shorten the learning curve period of SSRC and to be initiative for widening the indication for minimally invasive single site cholecystectomy. We also expect that further well-designed randomized prospective validation studies would confirm our early experiences with the reverse port technique.
ACKNOWLEDGEMENT
The authors sincerely thank Chang Hoon Lee (Video editor, Yonsei university Severance Hospital, Division of Media & Public Relations) for editing the video clips in this article. The authors also thank Dr. Hyung Seok Park, Department of Surgery, Yonsei University College of Medicine, for his advice about the cumulative sum analysis.
Footnotes
Abbreviations: ASA score = American Society of Anesthesiologists score, BMI = body mass index, CBD = common bile duct, CO2 = carbon dioxide, CUSUM = CUmulative SUM control chart, ICG = IndoCyanin Green, LC = laparoscopic cholecystectomy, R-LESS = robotic laparo-endoscopic single-site surgery, SILC = single incision laparoscopic cholecystectomy, SSRC = single-site robotic cholecystectomy, SSRC-O = single-site robotic cholecystectomy using the original single port group, SSRC-R = single-site robotic cholecystectomy using the reverse single port group.
Supplemental Digital Content is available for this article.
The authors have no funding and conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Perissat J, Collet D, Belliard R. Gallstones: laparoscopic treatment—cholecystectomy, cholecystostomy, and lithotripsy. Our own technique. Surg Endosc 1990; 4:1–5. [DOI] [PubMed] [Google Scholar]
- 2.Grace PA, Quereshi A, Coleman J, et al. Reduced postoperative hospitalization after laparoscopic cholecystectomy. Br J Surg 1991; 78:160–162. [DOI] [PubMed] [Google Scholar]
- 3.NIH Consensus Conference. Gallstones and laparoscopic cholecystectomy. JAMA 1993; 269:1018–1024. [PubMed] [Google Scholar]
- 4.Antoniou SA, Pointner R, Granderath FA. Single-incision laparoscopic cholecystectomy: a systematic review. Surg Endosc 2011; 25:367–377. [DOI] [PubMed] [Google Scholar]
- 5.Choi SH, Hwang HK, Kang CM, et al. Single-fulcrum laparoscopic cholecystectomy: a single-incision and multi-port technique. ANZ J Surg 2012; 82:529–534. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez AM, Rabaza JR, Donkor C, et al. Single-incision cholecystectomy: a comparative study of standard laparoscopic, robotic, and SPIDER platforms. Surg Endosc 2013; 27:4524–4531. [DOI] [PubMed] [Google Scholar]
- 7.Rivas H, Varela E, Scott D. Single-incision laparoscopic cholecystectomy: initial evaluation of a large series of patients. Surg Endosc 2010; 24:1403–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang HK, Choi SH, Kang CM, et al. Single-fulcrum laparoscopic cholecystectomy in uncomplicated gallbladder diseases: a retrospective comparative analysis with conventional laparoscopic cholecystectomy. Yonsei Med J 2013; 54:1471–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts KE, Solomon D, Duffy AJ, et al. Single-incision laparoscopic cholecystectomy: a surgeon's initial experience with 56 consecutive cases and a review of the literature. J Gastrointest Surg 2010; 14:506–510. [DOI] [PubMed] [Google Scholar]
- 10.Romanelli JR, Earle DB. Single-port laparoscopic surgery: an overview. Surg Endosc 2009; 23:1419–1427. [DOI] [PubMed] [Google Scholar]
- 11.Qiu Z, Sun J, Pu Y, et al. Learning curve of transumbilical single incision laparoscopic cholecystectomy (SILS): a preliminary study of 80 selected patients with benign gallbladder diseases. World J Surg 2011; 35:2092–2101. [DOI] [PubMed] [Google Scholar]
- 12.Kaouk JH, Goel RK, Haber GP, et al. Robotic single-port transumbilical surgery in humans: initial report. BJU Int 2009; 103:366–369. [DOI] [PubMed] [Google Scholar]
- 13.Uras C, Boler DE, Erguner I, et al. Robotic single port cholecystectomy (R-LESS-C): experience in 36 patients. Asian J Surg 2013. [DOI] [PubMed] [Google Scholar]
- 14.Vidovszky TJ, Carr AD, Farinholt GN, et al. Single-site robotic cholecystectomy in a broadly inclusive patient population: a prospective study. Ann Surg 2013. [DOI] [PubMed] [Google Scholar]
- 15.Pietrabissa A, Sbrana F, Morelli L, et al. Overcoming the challenges of single-incision cholecystectomy with robotic single-site technology. Arch Surg 2012; 147:709–714. [DOI] [PubMed] [Google Scholar]
- 16.Morel P, Pugin F, Bucher P, et al. Robotic single-incision laparoscopic cholecystectomy. J Robot Surg 2012; 6:273–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konstantinidis KM, Hirides P, Hirides S, et al. Cholecystectomy using a novel Single-Site® robotic platform: early experience from 45 consecutive cases. Surg Endosc 2012; 26:2687–2694. [DOI] [PubMed] [Google Scholar]
- 18.Buzad FA, Corne LM, Brown TC, et al. Single-site robotic cholecystectomy: efficiency and cost analysis. Int J Med Robot 2013; 9:365–370. [DOI] [PubMed] [Google Scholar]
- 19.Autorino R, Kaouk JH, Stolzenburg JU, et al. Current status and future directions of robotic single-site surgery: a systematic review. Eur Urol 2013; 63:266–280. [DOI] [PubMed] [Google Scholar]
- 20.Kroh M, El-Hayek K, Rosenblatt S, et al. First human surgery with a novel single-port robotic system: cholecystectomy using the da Vinci Single-Site platform. Surg Endosc 2011; 25:3566–3573. [DOI] [PubMed] [Google Scholar]
- 21.Wren SM, Curet MJ. Single-port robotic cholecystectomy: results from a first human use clinical study of the new da Vinci Single-Site surgical platform. Arch Surg 2011; 146:1122–1127. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Jung MJ, Hwang HK, et al. The first experiences of robotic single-site cholecystectomy in Asia: a potential way to expand minimally-invasive single-site surgery? Yonsei Med J 2015; 56:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg 1995; 180:101–125. [PubMed] [Google Scholar]
- 24.Callery MP. Avoiding biliary injury during laparoscopic cholecystectomy: technical considerations. Surg Endosc 2006; 20:1654–1658. [DOI] [PubMed] [Google Scholar]
- 25.Avgerinos C, Kelgiorgi D, Touloumis Z, et al. One thousand laparoscopic cholecystectomies in a single surgical unit using the “critical view of safety” technique. J Gastrointest Surg 2009; 13:498–503. [DOI] [PubMed] [Google Scholar]
- 26.Buchs NC, Pugin F, Azagury DE, et al. Real-time near-infrared fluorescent cholangiography could shorten operative time during robotic single-site cholecystectomy. Surg Endosc 2013; 27:3897–3901. [DOI] [PubMed] [Google Scholar]
- 27.Spinoglio G, Priora F, Bianchi PP, et al. Real-time near-infrared (NIR) fluorescent cholangiography in single-site robotic cholecystectomy (SSRC): a single-institutional prospective study. Surg Endosc 2013; 27:2156–2162. [DOI] [PubMed] [Google Scholar]
- 28.Buchs NC, Hagen ME, Pugin F, et al. Intra-operative fluorescent cholangiography using indocyanin green during robotic single site cholecystectomy. Int J Med Robot 2012; 8:436–440. [DOI] [PubMed] [Google Scholar]


