Abstract
Dioxin has been recognized as an environmental endocrine disruptor, but epidemiology studies of its effects on type 2 diabetes mellitus (DM) found inconsistent results, especially in men. Therefore, we conducted a study in Taiwan to evaluate the association between exposure to dioxin and DM.
We recruited participants in an area where the residents were exposed to dioxin released from a factory. Using 20 and 64 pg WHO98-TEQDF/g lipid as the cut-offs, we categorized participants into 3 groups according to the level of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) in the serum. We defined DM as a fasting plasma glucose level more than 126 mg/dl or an existing diagnosis.
Of the 2898 participants, 425 patients of DM were identified, and we observed positive associations between dioxin and DM. After adjusting for age and body mass index (BMI), we found that a high serum dioxin level was an independent risk factor for DM (adjusted odds ratio [AOR] associated with 20–63 pg WHO98-TEQDF/g lipid = 2.1, 95% confidence interval [95% CI] 1.5–2.9; AOR for ≥64 pg WHO98-TEQDF/g lipid = 3.2, 95% CI 2.1–4.8). The findings are compatible with those in previous studies of PCDD/Fs. When we stratified the participants by sex, the serum dioxin level remained an independent risk factor for DM in both men and women.
Exposure to dioxin is a risk factor for DM, independent of age and BMI in both men and women. Therefore, screening and intervention programs should be considered in endemic areas of exposure to dioxin.
INTRODUCTION
Dioxin has been recognized as an environmental endocrine disruptor for several decades, but the results from epidemiology studies of its effects on type 2 diabetes mellitus (DM) were inconsistent. Although a study in Germany found the prevalence of DM increased in the workers of a phenoxy herbicides factory who were exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) after a chemical reactor incident,1 a study of U.S. workers exposed to TCDD found no increased risk of DM.2 A study of U.S. veterans who were exposed to Agent Orange and its contaminant TCDD during the Vietnam War found higher prevalence of DM,3 but a re-analysis of the data did not find such an association.4 In Seveso, Italy, where exposure to substantial amounts of relatively pure TCDD occurred in a large population, excess mortality from DM was found in women, but not in men.5
In Taiwan, a 24-year follow-up of the Yucheng (“oil disease”) cohort, who were exposed to dioxin through ingestion of rice oil contaminated with polychlorinated biphenyls (PCBs), found an association between high dioxin levels and DM in women, but not in men.6 In a study of residents living near a factory that polluted the environment with PCDD/Fs, however, a high serum level of dioxin was found to be associated with an increased risk of insulin resistance after adjusting for sex, but the risk was lower in women.7 In animal experiments, TCDD was found to cause hypoinsulinemia in the rabbits and rats.8,9 It has been proposed that TCDD can lower the insulin production and secretion by beta cells through the reduction of glucose uptake caused by the decreased GLUT2 expression.10
Despite the facts that animal experiments have demonstrated the association between dioxin exposure and DM and that the association is biologically plausible, epidemiology studies observed inconsistent results. There are some hypotheses regarding reasons for the possible sex difference, including (1) men had lower exposure levels,6 (2) men had a higher prevalence of smoking, which activates the aryl-hydrocarbon receptor that might be associated with the accelerated excretion of PCBs,11 (3) women had a higher percentage of fat, which leads to a longer deposit of these lipophilic compounds, and (4) women have higher estrogen levels and PCDFs and some PCBs can induce CYP1A1 and CYP1B1 gene expression,12 which catalyze hydroxylation of the A-ring of estradiol to form the catechol estrogen 4-hydroxyl estradiols that may generate free radicals in their metabolism via redox-active compounds such as reactive semiquinone intermediates, and free radicals are known to cause increased oxidative stress, which is associated with DM.13 However, these hypotheses were still under debate, and the small number of DM cases (and thus limited study power) was also a plausible reason why these previous studies had inconsistent results. Therefore, we conducted a study in an area in Taiwan where the residents were exposed to dioxin released from environmental pollution to assess the effects of dioxin on DM and to identify related factors.
METHODS
Study Population
This study was carried out in the An-nan District of the Tainan City in southwestern Taiwan, where a factory produced pentachlorophenol (PCP) daily during the period between 1965 and 1979. PCDD/Fs are by-products of the PCP manufacturing, and a lot of chemicals were left at the original location after the factory was closed in 1982. The chemicals were washed out by the rainfall and entered into the sea reservoir, and a study found that the dioxin content of the sediment reached as high as 1000 to 6000 pg-TEQ/g dry weight.14 A series of studies have been conducted by our team, and the residents living in the vicinity of the deserted factory were found to have high daily intakes of PCDD/Fs from the food, especially seafood, and high serum PCDD/Fs levels in the blood.15–17 Residents of this area had an average serum PCDD/F level 3 times higher than those living in nonpolluted areas (62.5 versus 19.7 pg WHO98-TEQDF/g lipid).16 For exposure assessment, we adopted the serum levels measured by the Bureau of Health of the Tainan City between 2005 and 2007 using isotope dilution high-resolution gas chromatography/high-resolution mass spectrometry. The detailed methodology of the measurements has been described in previous reports.7,16,17 As a previous study showed that the average serum PCDD/Fs level in the general population of Taiwan typically ranged from 15 to 20 pg WHO98-TEQDF/g lipid,18 we define a “high dioxin level” as a serum PCDD/Fs level of at least 20 pg WHO98-TEQDF/g lipid. In addition, we used the value 64 pg WHO98-TEQDF/g lipid determined by the Tainan City government for compensation as another cut-off. Furthermore, we also tried analyzing the data with the dioxin level presented as quartiles.
Collection of Data
With the funding and assistance of the Tainan City government, we offered health examination to all residents above 18 years old in the exposure area. According to the results of health examination, we defined DM as a fasting blood sugar more than 126 mg/dl or a previous diagnosis of DM by a physician. In addition, we used 24 kg/m2 as the cut-off of body mass index (BMI) to define overweight.19 To control the effects of age, we stratified the participants using two cut-offs: 40 years, the age that the Taiwan government recommends starting routine health check-up, and 65 years, the age that above which the Taiwan government define as elderly.
In addition to health examination, we used a self-administrated questionnaire to collect data on demographic characteristics and medical history. The protocol of this study was approved by the Institution Review Board of the National Cheng Kung University Hospital. All the participants provided their written informed consent to participate in this study.
Statistical Analysis
To identify the risk factors for DM, we compared the prevalence of each potential risk factor between participants with and without DM and evaluated the differences using the Chi-square test. To evaluate the effects of risk factors, we performed univariate logistic regressions and calculated the odds ratio (OR) and associated 95% confidence interval (CI) for each variable. In addition, we performed multiple logistic regressions to identify independent predictors of DM and evaluate their effects. Variables with P values less than 0.15 in the univariate analyses were included in the multivariate model. An adjusted odd ratio (AOR) and the associated 95% CI were calculated for each risk factor. Furthermore, as previous studies showed inconsistent results for the effects of dioxin on DM between men and women, we conducted separate analyses for each sex. Whereas the duration of residency in the endemic area is related to the total dose of exposure and thus is a potential predictor of the outcome, it is correlated with age, and therefore we conducted a separate analysis to adjust for its effects. We analyzed the data using SPSS version 15.0.
RESULTS
Of the 3034 residents in the exposure area who were above 18 years old, we excluded 136 with incomplete data. Of the 2898 participants included in this study, 1143 had dioxin level between 20 and 63 pg WHO98-TEQDF/g lipid, and 284 had a dioxin level of at least 64 pg WHO98-TEQDF/g lipid. When we compared participants with DM with those who did not have DM, we observed differences in the serum dioxin level, age, and BMI, but not sex and family history of DM (Table 1). There are 339 (23.8%) DM cases in high dioxin exposure (dioxin level ≥20 pg WHO98-TEQDF/g lipid). Therefore, we performed further analyses of the data on dioxin level, age, and BMI.
TABLE 1.
Comparisons between Patients of Diabetes and the Normal Population
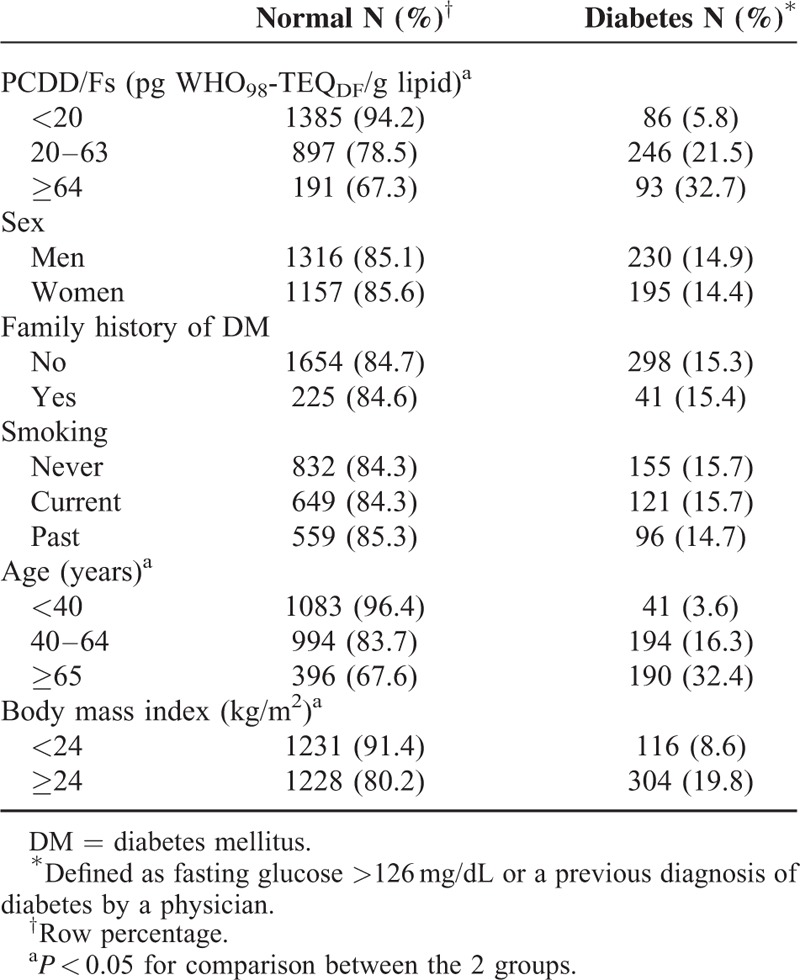
In the univariate logistic regression analyses, we found that a dioxin level between 20 and 63 pg WHO98-TEQDF/g lipid was associated with an OR of 4.4 (95% CI 3.4–5.7) and a dioxin level ≥64 pg WHO98-TEQDF/g lipid was associated with an OR of 7.8 (95% CI 5.6–10.9) for DM (Table 2). In addition to BMI, age between 40 and 64 years as well as age ≥65 years were both risk factors for DM. After adjusting for age and BMI in the multiple logistic regressions, we found a high dioxin level (AOR = 2.1, 95% CI 1.5–2.8 for 20–63 pg WHO98-TEQDF/g lipid and AOR = 2.7, 95% CI 1.9–4.0 for ≥64 pg WHO98-TEQDF/g lipid) was a risk factor for DM, independent of age and BMI (Table 2). When the dioxin level was included as quartiles in the model, AORs were 1.8 (95% CI 1.1–2.9), 2.9 (95% CI 1.7–4.8), and 3.4 (95% CI 2.0–5.8) for the second, third, and fourth quartiles with a positive trend (P < 0.001) (Table 2).
TABLE 2.
Risk Factors for Diabetes (N = 2898)
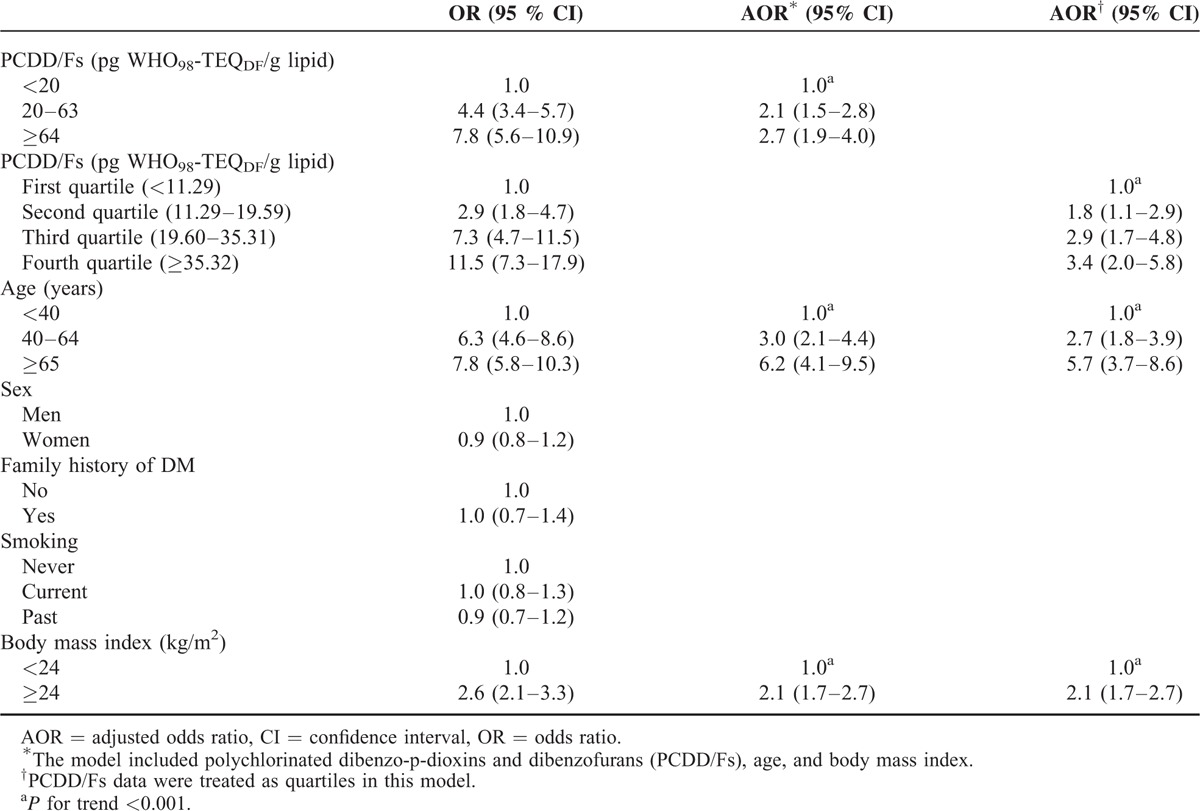
In the stratified analyses, we found that a high dioxin level was associated with DM (AOR = 2.3, 95% CI 1.6–3.3 for 20–63 pg WHO98-TEQDF/g lipid and AOR = 3.3, 95% CI 2.0–5.5 for ≥64 pg WHO98-TEQDF/g lipid) in men, independent of age and BMI (Table 3). Likewise, in women, a high dioxin level was associated with DM (AOR = 2.0, 95% CI 1.1–3.3 for 20–63 pg WHO98-TEQDF/g lipid and AOR = 2.3, 95% CI 1.2–4.4 for ≥64 pg WHO98-TEQDF/g lipid), independent of age and BMI. In both men and women, age and BMI were risk factors for DM, independent of the dioxin level (Table 3).
TABLE 3.
Risk Factors for Diabetes by Sex
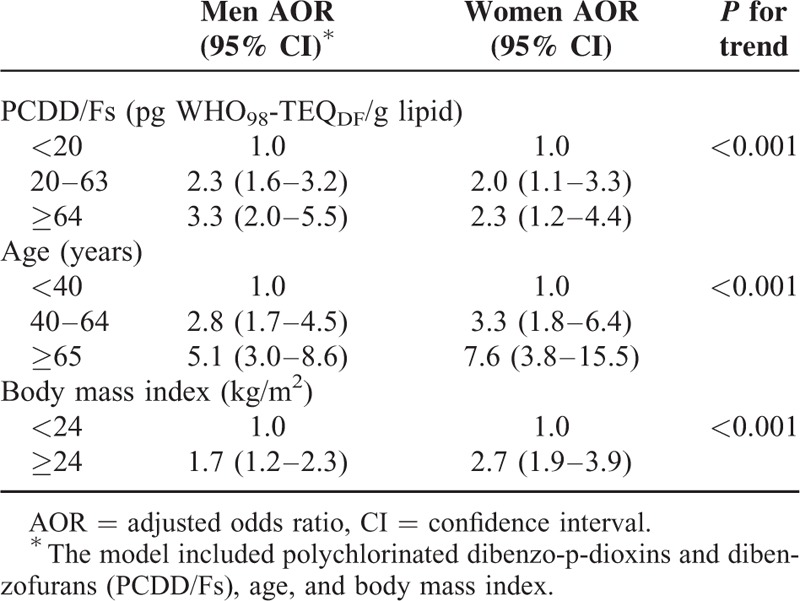
When we replaced age with the duration of residency in the endemic area in the model, a high dioxin level (AOR = 2.2, 95% CI 1.6–3.0 for 20–63 pg WHO98-TEQDF/g lipid and AOR = 3.6, 95% CI 2.4–5.4 for ≥64 pg WHO98-TEQDF/g lipid) remained a risk factor for DM, independent of duration of residency in the endemic area and BMI (Table 4). The duration of residency in the endemic area was also an independent risk factor for DM with a positive trend (P = 0.01) (Table 4).
TABLE 4.
Risk Factors for Diabetes with Residence Years (N = 2351)
DISCUSSION
In our study, we found that a high dioxin level (PCDD/Fs ≥20 pg WHO98-TEQDF/g lipid) was associated with DM, and this is compatible with the findings of many previous studies. Combining “diabetes” and “dioxin” as key words to search literature indexed in the PubMed, we identified 179 articles, of which 39 were original articles on epidemiology studies. Of the 39 articles, 14 had data on dioxin and DM, but some were on the same population. Whereas this search did not intend to cover all the published reports on this topic, and more reports could be retrieved using more vigorous methods, it should include most of them. The study populations can be largely categorized into 3 types: workers with occupational exposure,2 general populations with20 or without specific exposures,21–25 and victims of specific events.5,6,26,27 Two studies had data on exposure to PCDDs/Fs, and both were on general populations in Japan. One found an AOR of 2.21 (95% CI 1.02–5.04) associated serum levels ≥18.00 pg WHO-TEQ/g lipid, and it can be estimated that the AOR associated with the group ≥18.00 pg WHO-TEQ/g lipid compared with those with levels less than 18.00 pg WHO-TEQ/g lipid was around 2.03, which is compatible with the finding of an AOR of 2.1 associated with the exposure group “20–63 pg WHO-TEQ/g lipid” in comparison with the exposure group “<20 pg WHO-TEQ/g lipid” in our study. In the other study, the highest quartile of exposure was at least 15.05 pg WHO-TEQ/g lipid, and it can be estimated that this level was associated with an AOR around 2.0 in comparison with levels less than 15.05 pg WHO-TEQ/g lipid, which is also compatible with our findings. In these studies, as well as our study, the exposures in fact contained 2 persistent organic pollutants, but the 2 pollutants have similar chemical structure28 and share similar mechanisms of toxicity.29 Therefore, all these studies treated the 2 pollutants together, instead of studying them separately.
Findings on TCDD specifically were much less consistent. A study of U.S. workers exposed to TCDD did not find an increase in AOR even when the highest exposure group was compared with the lowest exposure group, with a difference of more than 10 folds.2 In the Seveso cohort, a much larger increase (the A Zone [477.0 ppt] and B Zone [94.0 ppt] combined versus the Reference [5.5 ppt]) was associated with an increased mortality rate ratio (MRR) in women (1.7), but not in men,30 and the estimated MRR was higher in the B Zone (1.4 versus 0.8), which had the lower exposure when the comparison was made between the 2 exposure zones.5 In fact, the estimated DM mortality rate in the B Zone was even lower than that in the Reference region (MRR = 0.8), although there was a 17-fold increase in the exposure level, which was compatible with the finding in the Seveso Women's Study that a 10-fold increase in the blood level was associated with an adjusted hazard ratio of 0.76.26 In studies on exposures to agent orange, however, a positive association between the blood TCDD level and DM was observed in both U.S.27 and Korean31 veterans (all men). Two reports from the National Health and Examination Survey of the U.S. observed positive effects of certain specific PCB on DM, one on 2,2’,4,4’,5,5’-hexachlorobiphenyl,32 and the other on 3,3’,4,4’,5-pentachlorobiphenyl.24 However, findings on levels of mixed PCBs were less consistent. Whereas a follow-up of the Yucheng cohort in Taiwan observed an increased risk in women but not in men,6 a study of the Great Lakes sport fish consumers cohort in the U.S. observed an increased risk in men but not in women.20 However, also on women in the U.S., the Nurses’ Health Study did not observe an association.22 In studies that observed men and women together, the results are more consistent. In the Prospective Investigation of the Vasculature in Uppsala Senior Study in Sweden21 and 2 studies in Japan,25 higher exposure levels were associated with higher risks.
In addition to decreasing the insulin production and secretion by the beta cells,33 another possible mechanism to explain the association is that TCDD can reduce glucose uptake by adipose tissue, liver, and pancreas. It is generally believed that the most toxic effects of dioxins and dioxin such as PCB congeners are mainly mediated by the aryl hydrocarbon receptor (AhR).34 Dioxin can activate the AhR and then suppress the function of peroxisome proliferator activated receptor (PPAR) γ, which may lead to insulin resistance.34,35 PPARs are ligand-activated transcription factors that control lipid metabolism and homeostasis, and PPARγ promotes differentiation of adipocytes and translation of the glucose transporter protein GLUT4.36,37 Therefore, dioxin exposure may progressively lower the translation of GLUT4 and cause insulin resistance and hyperglycemia.
Our study found that age is an important risk factor for DM, and this is compatible with the current knowledge that the prevalence of DM increases with age.38 Although an older age might indicate a longer duration of exposure, age remained a risk factor for DM independent of the dioxin level and BMI when we stratified the study population by the duration of residency in the endemic area (data not shown). The pathogenesis of DM can be classified into 2 pathways: insulin resistance and insulin secretary defects. The insulin secretary defect pathway seems most related to elder people.39 A study on environmental exposures found that PCB substantially increased the risk of DM in an elderly population,21 which is consistent with our finding.
Obesity is another well known important risk factors for DM.39,40 Although it is generally believed that people with high BMI tend to have more dioxin accumulated in the body, a study in Seveso study did not observe a significant association between the serum TCDD level and BMI in women.41 In our study, we found that a BMI of at least 24 kg/m2 was a risk factor for DM, independent of the dioxin level and age. When stratified by sex, it was found to be an independent risk factor for DM in both men and women.
In the 24-year follow-up study of Yuecheng cohort in Taiwan, high dioxin levels were found to be associated with DM in women (OR = 2.1, 95% CI 1.1–4.5), but not in men.6 Similar findings were observed in Seveso, Italy.5 In our study, when we stratified the by sex, we found an association between the dioxin level and DM in both men and women after adjusting for age and BMI. In comparison with our study, the Yuecheng cohort study had a much smaller number of participants and thus might not have enough study power to detect the difference. Likewise, whereas the study in Seveso had a larger population, there were only 26 cases of DM in total, while our study had 374 cases. We believe that the limited number of DM cases is a major reason why the previous studies had inconsistent results.
Although there is an update version of the WHO TFE, the 2005 WHO TEF, we used 1998 WHO TFE42 because the measurements were made using the 1998 unit. The updates were based on factors such as new toxicity values and the need to consider impurities in test compounds.43 The WHO 2005 TEF has the advantage of describing the level of uncertainty better, but the disadvantage is putting all data together and giving similar weights to all types of studies. Another difference is that the WHO 1998 TEF uses increments of 0.01, 0.05, 0.1, and so on, but the WHO 2005 TEF uses half order of magnitude increments on a logarithmic scale of 0.03, 0.1, 0.3, and so on. The 2005 WHO TEF value of a given sample may increase or decrease in comparison with the 1998 WHO TEF value, depending largely on the mixture being evaluated. Therefore, we cannot transfer the dioxin level directly from 1998 WHO TEF to 2005 WHO TEF.
Even with a large study population, our study has some limitations. We did not have data on some potential confounders such as habits of exercise, waist circumference, dietary intake, and socio-economic status. However, we had included BMI in the analyses, which is highly correlated with habits of exercise, waist circumference, and dietary intake, and thus can be regarded as a surrogate measurement for those factors. We used a 1-time measurement of dioxin level in the blood as the exposure indicator and did not have exact data on the cumulative exposure dose. Nonetheless, because the half-life of PCDD/Fs in the serum is as long as 7 years44,45 or longer 4,27 and the sources of the environment contamination remained similar during that period, we believe the level remained similar in a given participant over the years. In fact, most of the previous studies on the health effects of dioxin also had only one measurement over the study period. Furthermore, when we stratified the study population by the duration of residency in the endemic area, which is highly correlated with total exposure dose, the level of dioxin remained a risk factor for DM, independent of BMI. Although a family history is a well documented risk factor for DM, it was not identified as a significant risk factor in our study. We believe that this was mainly due to the inaccuracy of reported family history. This is an adult population, and therefore the parents and grandparents of the participants were mostly of very old ages. In the past, it was not uncommon that DM remained undiagnosed for the whole life in Taiwanese. In addition, some of the participants might not be informed by their family members of their DM histories. When the misclassification was not related to the exposure status (random misclassification), it would lead to an underestimation of the relative risk (biased towards the null), which is compatible with our case: the OR was higher than the null value (larger than 1) but did not reach statistical significance.
In the previous studies, we identified through the literature review that only a study in Seveso, Italy,5 has a larger study population, but the number of DM patients was smaller and there were no measurements of the exposure level on each participant. With a large population and a large number of DM patients, we could obtain more stable estimates of the risks and stratify the study population for further analyses. In particular, stratification by the duration of residency in the endemic area, which should be correlated with total exposure dose and age, can help demonstrate the effects of dioxin more clearly. With the actual measurement of the dioxin level on each participant in such a large population, we could obtain more accurate estimates of the risks than the previous studies.
In conclusion, we found that exposure to dioxin is a risk factor for DM, independent of age and BMI, in both men and women. Screening programs for identifying DM in its early stage and intervention programs for prevention and control of DM should be considered in endemic areas of exposure.
Acknowledgments
We would like to thank the colleagues in the Department of Environmental and Occupational Health and the Research Center for Environmental Trace Toxic Substances of the National Cheng Kung University as well as the Tainan City Government for helping us completing this study.
Footnotes
Abbreviations: BMI = body mass index, DM = diabetes mellitus, PCBs = polychlorinated biphenyls, PCDD/Fs = polychlorinated dibenzo-p-dioxins and dibenzofurans, TCDD = 2,3,7,8-tetrachlorodibenzo-p-dioxin.
C-CL and H-RG contributed equally to the work.
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Zober A, Ott MG, Messerer P. Morbidity follow up study of BASF employees exposed to 2,3,7,8-tetrachlorodibenzo-pdioxin (TCDD) after a 1953 chemical reactor incident. Occup Environ Med 1994; 51:479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calvert GM, Sweeney MH, Deddens J, et al. Evaluation of diabetes mellitus, serum glucose, and thyroid function among United States workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Occup Environ Med 1999; 56:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henriksen GL, Ketchum NS, Michalek JE, et al. Serum dioxin and diabetes mellitus in veterans of Operation Ranch Hand. Epidemiology 1997; 8:252–258. [DOI] [PubMed] [Google Scholar]
- 4.Kerger BD, Scott PK, Pavuk M, et al. Re-analysis of Ranch Hand study supports reverse causation hypothesis between dioxin and diabetes. Crit Rev Toxicol 2012; 42:669–687. [DOI] [PubMed] [Google Scholar]
- 5.Bertazzi PA, Consonni D, Bachetti S, et al. Health effects of dioxin exposure: a 20-year mortality study. Am J Epidemiol 2001; 153:1031–1044. [DOI] [PubMed] [Google Scholar]
- 6.Wang SL, Yang CY, Tsai PC, et al. Increased risk of diabetes and polychlorinated biphenyls and dioxins: a 24-year follow-up study of the Yucheng cohort. Diabetes Care 2008; 31:1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang JW, Ou HY, Chen HL, et al. Dioxin exposure and insulin resistance in Taiwanese living near a highly contaminated area. Epidemiology 2010; 21:56–61. [DOI] [PubMed] [Google Scholar]
- 8.Ebner K, Brewster DW, Matsumura F. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on serum insulin and glucose levels in the rabbit. J Environ Sci Health B 1988; 23:427–438. [DOI] [PubMed] [Google Scholar]
- 9.Gorski JR, Rozman K. Dose-response and time course of hypothyroxinemia and hypoinsulinemia and characterization of insulin hypersensitivity in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-treated rats. Toxicology 1987; 44:297–307. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura F. Mechanism of action of dioxin-type chemicals, pesticides, and other xenobiotics affecting nutritional indexes. Am J Clin Nutr 1995; 61:695S-F 701S. [DOI] [PubMed] [Google Scholar]
- 11.Safe SH. Development validation and problems with the toxic equivalency factor approach for risk assessment of dioxins and related compounds. J Anim Sci 1998; 76:134–141. [DOI] [PubMed] [Google Scholar]
- 12.Wang SL, Chang YC, Chao HR, et al. Body burdens of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls and their relations to estrogen metabolism in pregnant women. Environ Health Perspect 2006; 114:740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceriello A. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care 2008; 31:S181–S184. [DOI] [PubMed] [Google Scholar]
- 14.Chen HL, Su HJ, Liao PC, et al. Serum PCDD/F concentration distribution in residents living in the vicinity of incinerators and its association with predicted ambient dioxin exposure. Chemosphere 2004; 54:1421–1429. [DOI] [PubMed] [Google Scholar]
- 15.Chen HL, Su HJ, Lee CC. Patterns of serum PCDD/Fs affected by vegetarian regime and consumption of local food for residents living near municipal waste incinerators from Taiwan. Environ Int 2006; 32:650–655. [DOI] [PubMed] [Google Scholar]
- 16.Lee CC, Guo YL, Chang HY, et al. Human PCDD/PCDF levels near a pentachlorophenol contamination site in Tainan, Taiwan. Chemosphere 2006; 65:436–448. [DOI] [PubMed] [Google Scholar]
- 17.Lee CC, Lin WT, Liao PC, et al. High average daily intake of PCDD/Fs and serum levels in residents living near a deserted factory producing pentachlorophenol (PCP) in Taiwan: influence of contaminated fish consumption. Environ Pollut 2006; 141:381–386. [DOI] [PubMed] [Google Scholar]
- 18.Chen HL, Liao PC, Su HJ, et al. Profile of PCDD/F levels in serum of general Taiwanese between different gender, age and smoking status. Sci Total Environ 2005; 337:31–43. [DOI] [PubMed] [Google Scholar]
- 19.Pan WH, Flegal KM, Chang HY, et al. Body mass index and obesity-related metabolic disorders in Taiwanese and USA whites and blacks: implication for definitions of overweight and obesity for Asians. Am J Clin Nutr 2004; 79:31–39. [DOI] [PubMed] [Google Scholar]
- 20.Turyk M, Anderson HA, Knobeloch L, et al. Prevalence of diabetes and body burdens of polychlorinated biphenyls, polybrominated diphenyl thers, and p,p’-diphenyldichloroethene in Great Lakes sport fish consumers. Chemosphere 2009; 75:674–679. [DOI] [PubMed] [Google Scholar]
- 21.Lee DH, Lind PM, Jacobs DR, Jr, et al. Polychlorinated biphenyls and organochlorine pesticides in plasma predict development of type 2 diabetes in the elderly. Diabetes Care 2011; 34:1778–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu H, Bertrand KA, Choi AL, et al. Persistent organic pollutants and type 2 diabetes: a prospective analysis in the Nurses's health study and meta-analysis. Environ Health Perspect 2013; 121:153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamoto M, Arisawa K, Uemura H, et al. Association between blood levels of PCDDs/PCDFs/dioxin-like PCBs and history of allergic and other diseases in the Japanese population. Int Arch Occup Environ Health 2013; 86:849–859. [DOI] [PubMed] [Google Scholar]
- 24.Everett CJ, Thompson OM. Associations of dioxins, furans and dioxin-like PCBs with diabetes and pre-diabetes: is the toxic equivalency approach useful? Environ Res 2012; 118:107–111. [DOI] [PubMed] [Google Scholar]
- 25.Uemura H, Arisawa K, Hiyoshi M, et al. Associations of environmental exposure to dioxins with prevalent diabetes among general inhabitants in Japan. Environ Res 2008; 108:63–68. [DOI] [PubMed] [Google Scholar]
- 26.Warner M, Mocarelli P, Brambilla P, et al. Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: the Seveso women's health study. Environ Health Perspect 2013; 121:906–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michalek JE, Pavuk M. Diabetes and cancer in veterans of Operation Ranch Hand after adjustment for calendar period, days of spraying, and time spent in Southeast Asia. J Occup Environ Med 2008; 50:330–340. [DOI] [PubMed] [Google Scholar]
- 28.Rappe C. Sources of PCDDs and PCDFs. Chemosphere 1992; 25:41–44. [Google Scholar]
- 29.Landers JP, Bunce NJ. The Ah receptor and the mechanism of dioxin toxicity. Biochem J 1991; 276:273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pesatori AC, Consonni D, Bachetti S, et al. Short- and long-term morbidity and mortality in the population exposed to dioxin after the “Seveso accident”. Ind Health 2003; 41:127–138. [DOI] [PubMed] [Google Scholar]
- 31.Kim JS, Lim HS, Cho SI, et al. Impact of agent orange exposure among Korean Vietnam veterans. Ind Health 2003; 41:149–157. [DOI] [PubMed] [Google Scholar]
- 32.Lee DH, Toscano W, Lee IK, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes. Diabetes Care 2006; 29:1638–1644. [DOI] [PubMed] [Google Scholar]
- 33.Novelli M, Piaggi S, De Tata V. 2,3,7,8-Tetrachlorodibenzo-p-dioxin induced impairment of glucose stimulated insulin secretion in isolated rat pancreatic islets. Toxicol Lett 2005; 156:307–314. [DOI] [PubMed] [Google Scholar]
- 34.Nebert DW, Dalton TP, Okey AB, et al. Role of aryl hydrocarbon receptor- mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem 2004; 279:23847–23850. [DOI] [PubMed] [Google Scholar]
- 35.Alexander DL, Ganem LG, Fernandez-Salguero P, et al. Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J Cell Sci 1998; 111:3311–3322. [DOI] [PubMed] [Google Scholar]
- 36.Remillard RBJ, Bunce NJ. Linking dioxin to diabetes: epidemiology and biologic plausibility. Environ Health Perspect 2002; 110:853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimaya A, Kursoki E, Shiduka K, et al. YM268 Increases the glucose uptake, cell differentiation, and mRNA expression of glucose transporter in 3T3-L1 adipocytes. Horm Metab Res 1998; 30:543–548. [DOI] [PubMed] [Google Scholar]
- 38.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes. Estimate for the year 2000 and projections for 2030. Diabetes Care 2004; 27:1047–1053. [DOI] [PubMed] [Google Scholar]
- 39.Hillier TA, Pedula KL. Characteristics of an adult population with newly diagnosis of type 2 diabetes. Diabetes Care 2001; 24:1522–1527. [DOI] [PubMed] [Google Scholar]
- 40.Leibson CL, Smith SA, Williamson DF, et al. Temporal trends in BMI among adults with diabetes. Diabetes Care 2001; 24:1584–1589. [DOI] [PubMed] [Google Scholar]
- 41.Consonni D, Pesatori AC, Zocchetti C, et al. Mortality in population exposured to dioxin after the seveso, Italy, accident in 1976: 25 years of follow-up. Am J Epidemiol 2008; 167:847–858. [DOI] [PubMed] [Google Scholar]
- 42.Van den Berg M, Birnbaum LS, Bosveld ATC, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect 1998; 106:775–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van den Berg M, Birnbaum LS, Denison M, et al. The 2005 World Health Organization re-evaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci 2006; 93:223–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sweeney MH, Calvert GA, Egeland GM, et al. Review and update on the results of the NIOSH medical study of workers exposed to chemicals contaminated with 2,3,7,8-tetrachlorodibenzodioxin. Teratog Carcinog Mutagen 1997-1998; 17:241–247. [PubMed] [Google Scholar]
- 45.Paustenbach DJ, Kerger BD. The University of Michigan Dioxin Exposure Study: estimating residential soil and house dust exposures to young children. Chemosphere 2013; 91:200–204. [DOI] [PubMed] [Google Scholar]


