Significance
Understanding how and why communities vary in their responses to climatic change is critical for global biodiversity conservation. In a 5-y experimental study of a heterogeneous grassland system, we found that the most nutrient-limited communities were relatively insensitive to alterations in rainfall, but that this insensitivity could be reversed by nutrient addition, leading to greatly enhanced productivity, near-complete species turnover, and accelerated diversity loss. Species with slow-growing, resource-conserving traits were especially vulnerable to decline. These results highlight that the ecological principle of colimitation by water and nutrients can lead to powerful predictions about climate change impacts on natural communities.
Keywords: biodiversity, climate change, resource colimitation, functional traits, low-productivity ecosystems
Abstract
Ecological theory and evidence suggest that plant community biomass and composition may often be jointly controlled by climatic water availability and soil nutrient supply. To the extent that such colimitation operates, alterations in water availability caused by climatic change may have relatively little effect on plant communities on nutrient-poor soils. We tested this prediction with a 5-y rainfall and nutrient manipulation in a semiarid annual grassland system with highly heterogeneous soil nutrient supplies. On nutrient-poor soils, rainfall addition alone had little impact, but rainfall and nutrient addition synergized to cause large increases in biomass, declines in diversity, and near-complete species turnover. Plant species with resource-conservative functional traits (low specific leaf area, short stature) were replaced by species with resource-acquisitive functional traits (high specific leaf area, tall stature). On nutrient-rich soils, in contrast, rainfall addition alone caused substantial increases in biomass, whereas fertilization had little effect. Our results highlight that multiple resource limitation is a critical aspect when predicting the relative vulnerability of natural communities to climatically induced compositional change and diversity loss.
Current and predicted climatic changes are expected to considerably alter the water balance experienced by terrestrial ecosystems. Climate change can lead to increases or decreases in mean annual rainfall, shifts in seasonal and annual rainfall variability, changes in the frequency and magnitude of extreme precipitation events, shifts from snow to rain, declining snowpack, and temperature-driven increases in the climatic water deficit (1–3). These water-related changes are expected to exert dramatic impacts on the primary productivity, species composition, trophic relationships, diversity, and ecosystem functioning of natural communities (4–8), especially in arid and semiarid ecosystems (9–11). Water-related climatic changes are expected to outweigh the direct effects of increased temperatures on many natural communities (12). Despite the pervasiveness of the projected ecological impacts of changed water availability, very little is known about the factors that make some natural communities more vulnerable or resistant than others (13, 14). An important step toward improving downscaled forecasts of climate change impacts on natural communities is to test explicit, theory-based predictions about the effects of altered water availability.
Because water is a resource for plants, the concept of limiting resources is a potentially important principle for making successful predictions about altered water availability. Classically, “Liebig’s law of the minimum” suggests that plant productivity is limited by the single resource that is in scarcest supply relative to demand (15). This theory was developed for agricultural systems, and although its simple logic is appealing, its appropriateness to complex natural communities and ecosystems has been questioned (16, 17). Growing theory and evidence suggest a newer principle, namely that multiple scarce resources may act simultaneously and synergistically to limit plant community productivity (17–21). Interactions among multiple limiting resources are much more commonly found in factorial resource addition experiments than predicted by Liebig’s law (17, 20, 22). For example, water availability alone may have very little impact on primary productivity if nutrients are in short supply (23–26). Multiple biochemical and physical mechanisms may underlie such resource interactions. For example, nutrient ion solubility and microbial mediation of nutrient cycling are tightly linked to water availability (27, 28), providing likely mechanisms for positive synergisms between water and nutrient supply.
One potential implication of the theory of multiple limiting resources is that where water and nutrients are jointly limiting to community productivity and composition, a given change in climate may have the strongest effects on fertile soils and the weakest effects where soil fertility is low. A variety of evidence is consistent with this prediction (29). For example, effects of experimental warming and drought were lower in an infertile limestone grassland than in a fertile ex-cultivated grassland (13, 30); post-Pleistocene vegetation change was less pronounced on infertile peridotite than in forests on fertile granitic substrates (31); and in our study system, both experimental watering (25, 32) and ambient variation in annual precipitation (33, 34) had less effect on grasslands on serpentine soils than on more productive grasslands on sedimentary soils. If these contrasting plant community responses to climate are attributable to colimitation by water and nutrients, then several important implications follow. First, the endemic-rich plant communities found on nutrient-poor soils worldwide (35) may be relatively secure in the face of climate change. Second, communities on low-nutrient soils may be exceptionally vulnerable to biodiversity loss under the synergistic impacts of climate change and anthropogenic nutrient addition (17).
Water and nutrient colimitation has yet to be tested as a cause for variable responses of natural communities to climate change. In conducting such a test, an additional factor that must be considered is the prevalence on unproductive soils of plant species with “resource-conservative” functional traits (e.g., short stature, low specific leaf area, and low tissue nitrogen concentration). Such traits confer tolerance to low nutrients at the cost of low maximal growth rates under resource-rich conditions (36–38). The prevalence of species with resource-conservative traits, rather than (or in addition to) nutrient limitation itself, may limit the responses of species and communities on infertile soils to altered water availability. Conversely, the dominance of species having the opposite (“resource-demanding,” “fast growing”) functional traits might render communities in fertile habitats more responsive to both water and nutrient addition. The theory of functional trait syndromes also enables predictions about how communities will change if nutrient and water addition coincides with the arrival of propagules of species with fast-growing functional traits. The expected result is a disproportionate loss of species with resource-conservative traits that are characteristic of unique endemic-rich floras of many low-nutrient substrates around the world (35, 39).
In a 5-y field experiment, we used factorial additions of water and full-spectrum (macro- and micro) nutrients to test whether nutrient addition would render the most unproductive and diverse communities more sensitive to water, as predicted under resource colimitation. Alternatively, the responsiveness of these communities to water might remain constrained by the absence of species with “fast-growing” functional trait syndromes. Our experimental system is a semiarid annual grassland in which fertile sedimentary soils and infertile (N- and Ca-poor) serpentine soils are interspersed over short distances (Fig. S1). Previous work showed that water addition alone had little effect on grassland communities on the infertile soils (25, 32), whereas fertilization alone had stronger effects (40–42). In this study, we examined a gradient comprising three habitats: “harsh serpentine” grassland with coarse rocky soils, high native diversity, and very low biomass; “lush serpentine” grassland with fine-textured alluvial soils and intermediate biomass and species composition; and “nonserpentine” grassland with sedimentary soils and high biomass of mainly exotic species (Table S1).
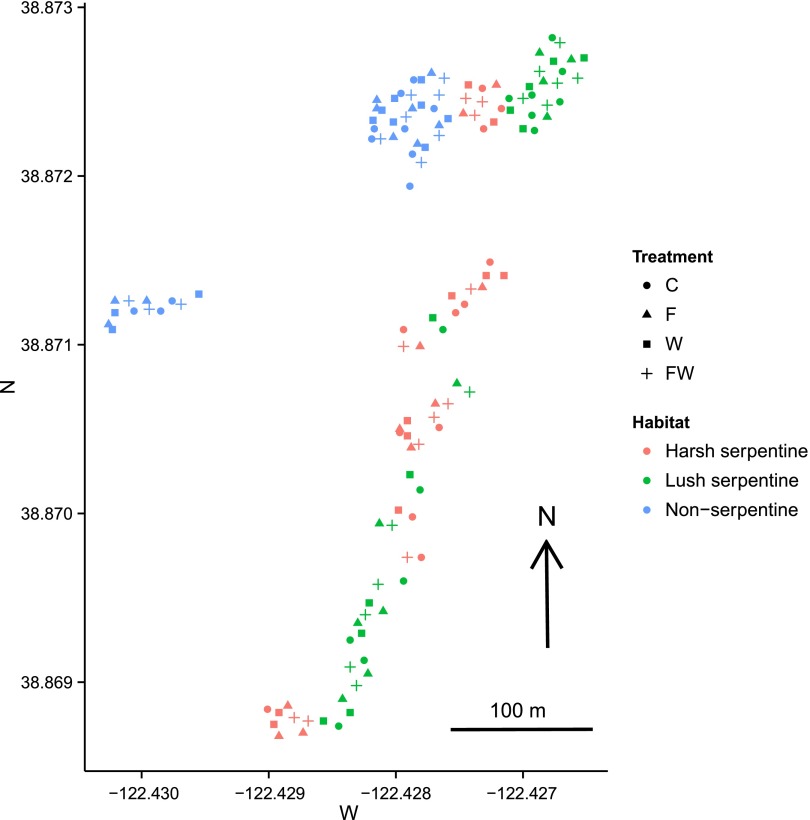
Fig. S1.
Figure of 131 experimental plots which were distributed among multiple alternating areas of harsh and lush serpentine grassland and two areas of nonserpentine grassland within a roughly 12-ha site. Water was brought to the area by an irrigation system consisting of nine watering lines that passed through multiple patches of the three grassland habitats. Color codes: red, harsh serpentine grasslands; green, lush serpentine grasslands; blue, nonserpentine grasslands. C, control; F, fertilization; FW, fertilization and watering; W, watering.
Table S1.
Soil and plant community variables (means ± SE) illustrating differences among harsh, lush, and nonserpentine grassland habitats
| Variable | Mean ± SE | F | P | ||
| Harsh serpentine | Lush serpentine | Nonserpentine | |||
| Ca/Mg ratio | 0.41 ± 0.04 | 0.37 ± 0.01 | 2.06 ± 0.08 | 41.42,129 | <0.001 |
| NH4+ (ppm) | 1.33 ± 0.07 | 2.65 ± 0.11 | 2.74 ± 0.10 | 80.82,126 | <0.001 |
| OM (%) | 2.18 ± 0.08 | 4.07 ± 0.12 | 3.81 ± 0.13 | 102.02,129 | <0.001 |
| pH | 7.21 ± 0.04 | 7.06 ± 0.03 | 6.10 ± 0.04 | 279.72,129 | <0.001 |
| Moisture retention capacity (%) | 21.32 ± 0.69 | 32.11 ± 0.60 | 26.75 ± 0.52 | 77.312,126 | <0.001 |
| Total biomass (g/0.0625 m2) | 8.64 ± 1.21 | 38.32 ± 2.47 | 38.37 ± 3.59 | 66.72,63 | <0.001 |
| Species richness (number/1 m2) | 14.91 ± 0.90 | 12.50 ± 0.74 | 9.81 ± 0.88 | 9.02,64 | <0.001 |
| Exotic cover (%) | 2.86 ± 1.14 | 59.26 ± 4.49 | 91.53 ± 2.70 | 188.72,64 | <0.001 |
| Native cover (%) | 73.47 ± 4.82 | 26.79 ± 5.63 | 2.87 ± 0.76 | 62.72,64 | <0.001 |
Differences between the habitats were tested using ANOVA. The soil samples were collected in early April 2010, just before the first treatment application. Plant biomass samples (including live biomass and litter) were collected and species richness and percentage covers of exotic and native species were estimated at the end of May 2010, using only untreated plots. Biomass and NH4+ concentration were square root-transformed, and Ca/Mg ratio and organic matter (OM) content were log-transformed for the analyses. Soil moisture retention capacity was measured under a constant 0.3 ATM pressure potential. The analyses were performed at A&L Western Laboratory (Ca/Mg ratio, OM, and pH) and at the University of California, Davis Analytical Laboratory (NH4+ and moisture-retention capacity).
We tested for synergistic effects of water and nutrient addition on community biomass, which we predicted would be strongest in the least-productive and most-diverse habitat, and on associated species turnover and decline in community diversity. To assess whether plant functional traits confer greater stability on low-productivity systems, we measured relevant traits [specific leaf area (SLA), height, carbon:nitrogen (C:N) ratio, leaf water content (LWC)] and used them as predictors of whole-community biomass. We also used the same traits to predict the probability that individual species will either decrease or increase under the treatments. We chose these traits because they are strongly linked to nutrient and water use (36, 38, 43, 44).
Results
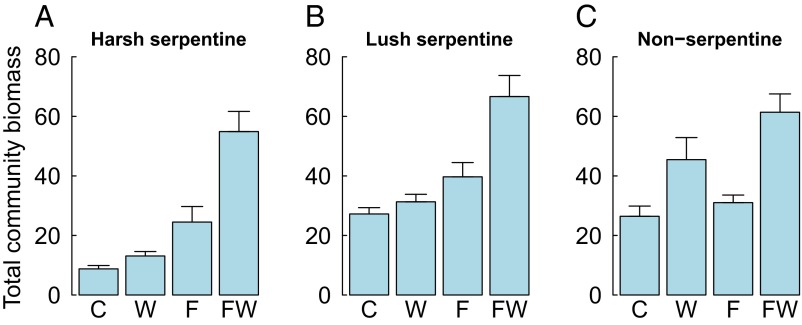
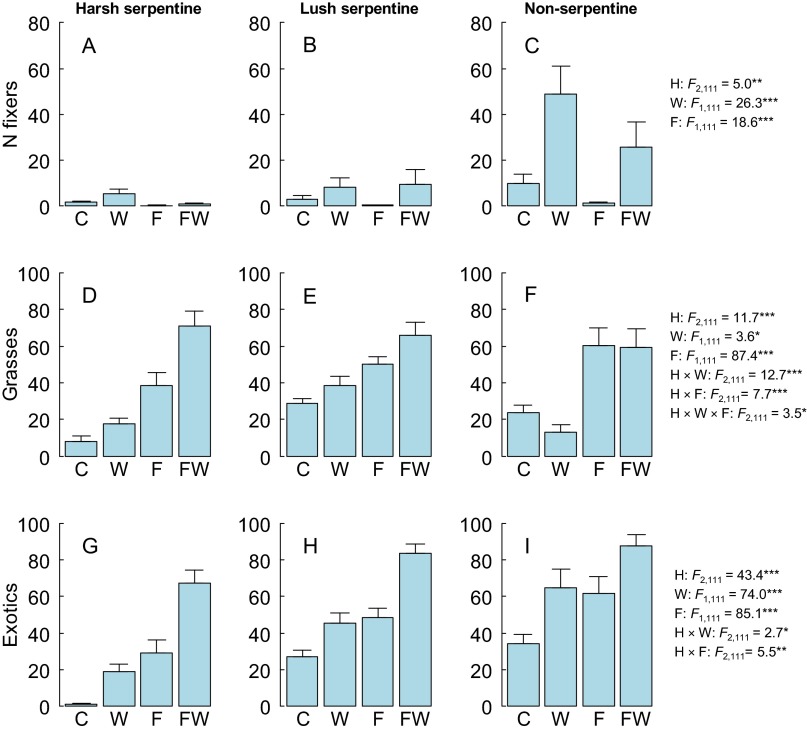
As predicted under water and nutrient colimitation, water and nutrient addition had synergistic effects on total community biomass [W × F interaction in linear mixed effects (LME)] (Table 1). This interaction appeared strongest in the harsh serpentine grassland habitat (Fig. 1A), although the H × W × F interaction was not significant (Table 1). In harsh serpentine grassland, biomass increased by 44% in watered-only plots, by 167% in fertilized-only plots, and by 511% (roughly equaling that of the two more fertile habitats) in plots receiving both water and nutrient addition (Fig. 1A and Fig. S2). Nutrient-only addition had smaller but significant positive effects on biomass across all habitats (Fig. 1 B and C and Table 1). As predicted, the positive impact of watering alone was highest in nonserpentine grassland, but it had weaker effects in harsh and lush serpentine grasslands (Fig. 1 and Table 1). The increase in biomass in nonserpentine grassland was mainly caused by exotic N-fixers that were relatively unresponsive to watering in harsh and lush serpentine grasslands (Fig. S3). The above results for total (live plus litter) biomass were qualitatively unchanged when live and litter biomasses were examined separately. Biomass increase in harsh serpentine grassland correlated strongly with increasing cover by exotic species (r = 0.7, t40 = 6.3, P < 0.0001). Grasses and exotic species responded strongly to the synergistic effects of water and nutrient addition in harsh serpentine grasslands (Fig. S3). In contrast, exotics also responded to watering alone and grasses mainly to fertilization alone in lush serpentine and nonserpentine grasslands (Fig. S3).
Table 1.
Results of LME models
| Effect | Total biomass | Simpson diversity | Species turnover | |||
| F | P | F | P | F | P | |
| Habitat (H) | 21.82,109 | <0.0001 | 16.72,111 | <0.0001 | 37.52,111 | <0.0001 |
| Watering (W) | 71.61,109 | <0.0001 | 0.31,111 | 0.5730 | 9.91,111 | 0.0022 |
| Fertilization (F) | 35.01,109 | <0.0001 | 15.81,111 | 0.0001 | 3.01,111 | 0.0837 |
| H × W | 2.82,109 | 0.0623 | 0.42,111 | 0.7009 | 0.32,111 | 0.7598 |
| H × F | 2.42,109 | 0.0984 | 0.32,111 | 0.7245 | 25.52,111 | <0.0001 |
| W × F | 5.21,109 | 0.0245 | 0.61,111 | 0.4551 | 0.41,111 | 0.5362 |
| H × W × F | 1.62,109 | 0.2036 | 3.72,111 | 0.0290 | 4.02,111 | 0.0216 |
Results of LME testing the effects of watering, fertilization, habitat, and their interactions on total community biomass (square root-transformed), Simpson diversity and species turnover (Bray–Curtis dissimilarity) in each plot among 2010 and 2014. All results with P ≤ 0.06 are in boldface.
Fig. 1.
Total biomass (g/0.0625 m2) with respect to different combinations of watering and fertilization after 5 y of the start of the experiment (in 2014) in harsh (A), lush (B), and nonserpentine (C) grasslands. C, control; F, fertilization; W, watering; FW, fertilization and watering.
Fig. S2.
Experimental rainfall and nutrient addition plots (indicated by white polygons) in harsh serpentine grassland. The watered and fertilized harsh serpentine plots have started to resemble nonserpentine grassland (foreground).
Fig. S3.
Cover of N-fixers (A–C), grasses (D–F), and exotics (G–I) under different combinations of watering and fertilization in harsh, lush, and nonserpentine plots. Treatment significances refer to results of LME models where the cover of each functional group was explained by habitat, watering, fertilization, and their interactions, and plots were nested within lines. Grasses were log-transformed, N-fixers log (x + 0.1) –transformed, and exotics square root-transformed for the analyses. F, fertilization; FW, fertilization and watering; H, habitat; W, watering. Significance codes: ***P < 0.001, **P < 0.01, *P < 0.07. Only significant (P ≤ 0.07) results are shown.
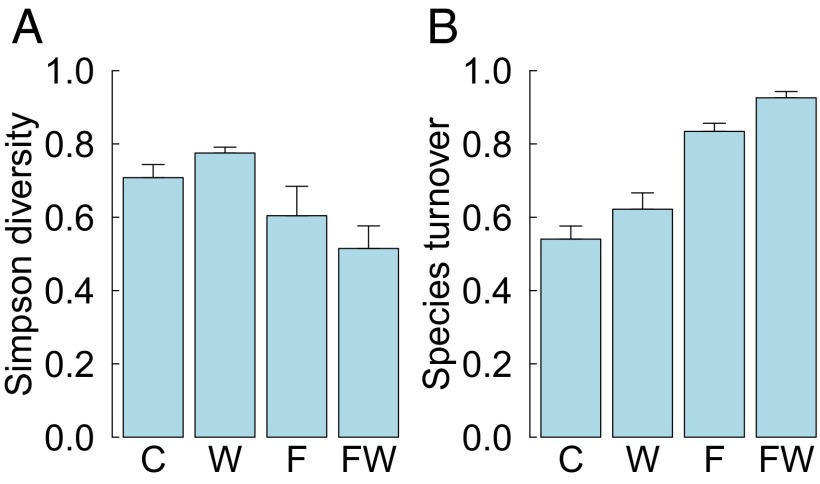
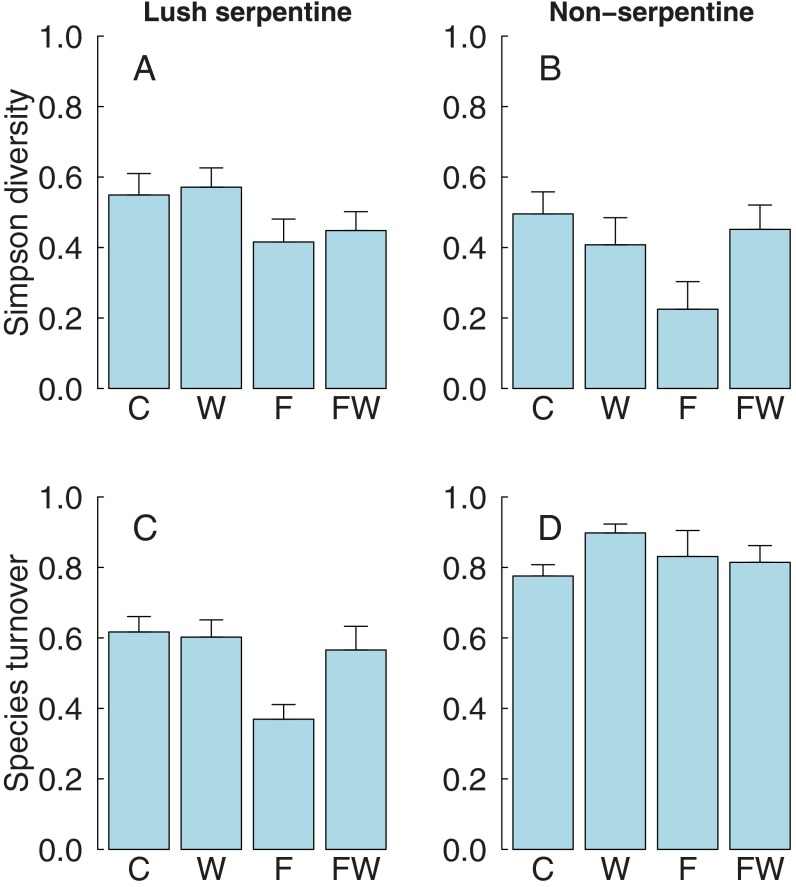
Community diversity, as measured by the abundance-weighted Simpson Index, declined in response to the combined water-nutrient treatment in the infertile harsh serpentine grassland where diversity had been initially highest (three-way H × W × F interaction (Fig. 2 and Table 1). This decline in diversity was associated with the increases in community biomass (r = −0.54, t40 = −4.1, P = 0.0002) and exotic cover (r = −0.56, t41 = −4.4, P < 0.0001). In the two more productive habitats, diversity declined in response to fertilization but not in response to watering or the combined water-nutrient treatment (Fig. S4). Results using other diversity measures (Shannon diversity, Simpson dominance, Pielou evenness) were qualitatively similar.
Fig. 2.
Simpson diversity (A) and turnover of species composition (B) (measured as Bray–Curtis dissimilarity among plots in 2010 and 2014) in harsh serpentine grasslands. C, control; F, fertilization; W, watering; FW, fertilization and watering.
Fig. S4.
Simpson diversity (A and B) and turnover of species composition (C and D) (measured as Bray–Curtis dissimilarity among plots in 2010 and 2014) in lush and nonserpentine grasslands. C, control; F, fertilization; FW, fertilization and watering; W, watering.
Within the harsh serpentine grassland, the simultaneous increase in biomass and decrease in diversity in the combined water-nutrient treatment were accompanied by nearly complete species turnover (measured as Bray–Curtis dissimilarity in species composition between 2010 and 2014, three-way H × W × F interaction) (Fig. 2, Table 1, and Fig. S2). This turnover of species composition correlated strongly with the cover of exotics (r = 0.7, t41 = 6.4, P < 0.0001) and with total biomass (r = 0.67, t40 = 5.8, P < 0.0001) in 2014. Species turnover showed weaker treatment responses in the two more productive habitats, with a negative fertilization-only effect in lush serpentine grassland and a slight positive watering-only effect in nonserpentine grassland, and no response to the combined water-nutrient treatment in either habitat (Table 1 and Fig. S4).
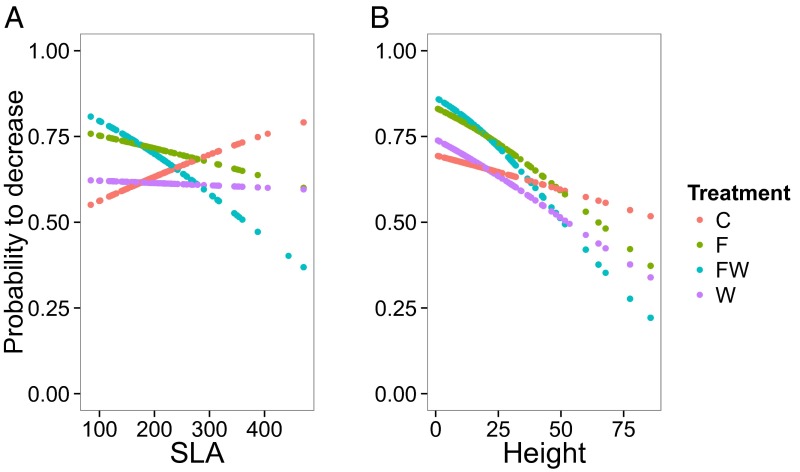
We found no evidence that community responses to resource additions were constrained by the pretreatment functional trait composition of the communities. There were no significant interactive effects of pretreatment functional trait values and experimental treatments on posttreatment biomass (Table S2). However, we did find that functional traits did predict how individual species responded to the treatments. In particular, shorter-statured species and species with low SLA were more likely to decrease in the combined water-nutrient treatment than other species (significant W × SLA and F × SLA interactions, z = −3.0, P = 0.003; z = 3.7, P < 0.001, respectively, and significant W × height and F × height interactions, z = −2.4, P = 0.016; z = 3.4, P < 0.001) (Fig. 3). We did not find significant effects for LWC or C:N ratio (Table S3).
Table S2.
Results of LME models testing interactions among watering, fertilization, and pretreatment (2010) CWM traits on total community biomass (square root-transformed)
| Variable | CWM SLA | CWM height | CWM C:N ratio | CWM LWC | ||||
| F | P | F | P | F | P | F | P | |
| Habitat (H) | 24.52,105 | <0.0001 | 24.52,105 | <0.0001 | 24.22,105 | <0.0001 | 24.32,105 | <0.0001 |
| Watering (W) | 54.11,105 | <0.0001 | 54.11,105 | <0.0001 | 53.41,105 | <0.0001 | 53.71,105 | <0.0001 |
| Fertilization (F) | 69.81,105 | <0.0001 | 69.81,105 | <0.0001 | 68.91,105 | <0.0001 | 69.31,105 | <0.0001 |
| Trait (see column) | <0.11,105 | 0.9607 | 0.81,105 | 0.3823 | <0.11,105 | 0.7921 | 1.01,105 | 0.3126 |
| H × W | 1.22,105 | 0.3104 | 1.22,105 | 0.3042 | 1.22,105 | 0.3118 | 1.12,105 | 0.3258 |
| H × F | 6.31,105 | 0.0026 | 6.11,105 | 0.0032 | 6.11,105 | 0.0030 | 5.91,105 | 0.0036 |
| W × F | 10.01,105 | 0.0020 | 9.91,105 | 0.0021 | 9.91,105 | 0.0021 | 10.21,105 | 0.0019 |
| W × Trait | <0.11,105 | 0.8148 | 0.61,105 | 0.4353 | <0.11,105 | 0.8582 | <0.11,105 | 0.8502 |
| F × Trait | <0.11,105 | 0.9209 | 0.31,105 | 0.5759 | <0.11,105 | 0.8841 | <0.11,105 | 0.8454 |
| H × W × F | 0.62,105 | 0.5487 | 0.72,105 | 0.4919 | 0.52,105 | 0.5825 | 0.52,105 | 0.5787 |
| W × F × Trait | 1.41,105 | 0.2457 | <0.11,105 | 0.9781 | <0.11,105 | 0.9245 | <0.11,105 | 0.8602 |
The interactions among habitat and treatments were included in all models. The impact of each trait on total community biomass (response variable in every model) was tested in its individual model to avoid multicollinearity. All significant effects (P ≤ 0.05) are in boldface.
Fig. 3.
Probability of individual species to decrease from 2010 to 2014 as a function of species specific (A) SLA (mm2), and (B) height (cm), under different combinations of watering and fertilization across all habitats. The values represent fitted (predicted) values per individual trait values from GLMM where three-way interactions among watering, fertilization, and traits (SLA and height in separate models) were used as explanatory variables, and likelihood of each species decreasing in each plot was used as response variable (each species was assigned 1 if it decreased and 0 if it did not, and this 1–0 variable was the response variable). In the models, species were nested within plots. Only fitted values but no data points are shown (those would be 1 s and 0 s). For clarity of the figures, we removed two extreme observations from SLA and one extreme observation from height data; however, parameter significances remained similar regardless of whether or not the extreme values were included in the models. C, control; F, fertilization; W, watering; FW, fertilization and watering.
Table S3.
Results of GLMM models using binomial error structure and testing the interactions among watering, fertilization, and individual species’ traits on the likelihood to each species to decrease/increase
| Variable | SLA | Height | C:N ratio | LWC | ||||
| z | P | z | P | z | P | z | P | |
| Watering (W) | 1.91 | 0.0560 | 1.37 | 0.1710 | −2.35 | 0.0187 | −2.20 | 0.0280 |
| Fertilization (F) | −4.48 | <0.0001 | −4.56 | <0.0001 | −3.92 | 0.0058 | −2.35 | 0.0186 |
| Trait (see column) | −1.54 | 0.1248 | −5.55 | <0.0001 | −0.94 | 0.3499 | NS | NS |
| W × Trait | −2.45 | 0.0143 | −2.41 | 0.0158 | 1.72 | 0.0848 | NS | NS |
| F × Trait | 3.66 | 0.0003 | 3.39 | 0.0007 | NS | NS | NS | NS |
Each species in each plot obtained value 1 if it decreased from 2010 to 2014, and value 0 if it increased. In the models, individual species were nested within plots (i.e., plot was used as a random variable). Each trait was tested in its individual model to avoid multicollinearity. We used manual models simplification to identify the most parsimonious models and used χ test to assess the significance of each explanatory variable (at the end, the models included only significant explanatory variables with P < 0.05).
Discussion
Our study adds soil fertility to a list of other factors, such as habitat fragmentation (45) and strong temperature limitation (46), which can render ecological communities especially vulnerable to climate change. Whereas several previous studies have reported relatively high resistance to climatic changes in unproductive ecosystems (13, 14, 30, 47, 48), ours is among the first to identify resource colimitation as the mechanism responsible for this resistance.
We found that colimitation by water and nutrients was responsible for constraining the response of the most infertile grassland habitats to an increase in rainfall. Water- and nutrient-only additions slightly enhanced biomass; however, the addition of both water and nutrients led to strong synergy, so that the biomass of the formerly infertile habitat equaled that of the more fertile grassland habitats. These findings support the predictions of multiple resource limitation theory (17–20), and not the traditional theory of single limiting resources (15, 17, 22). The synergistic biomass increase in the most infertile grassland habitat was accompanied by nearly complete species turnover and decreased diversity to match the level of the least diverse nonserpentine grassland habitat. Decreased diversity under multiple resource amendments is consistent with the niche dimension hypothesis, which proposes that decreasing the number of limiting resources should lead to loss of diversity (24, 49). Our results show that colimitation by nutrients and water is critical to the maintenance of high plant diversity on infertile soils, where the impacts of climate change may be less pronounced than in fertile ecosystems. However, if nutrient scarcity is relaxed (for example, because of high anthropogenic nutrient deposition), our results imply that dramatic increases in productivity, shifts in species composition, and loss of diversity will result.
Water addition alone had the greatest impact on biomass in the nonserpentine grasslands, where it caused a particularly marked increase of N-fixers, especially the exotic Vicia villosa. This impact matches our predictions as nonserpentine grassland is the most fertile habitat, where water addition alone should have the greatest impact. However, we did not see a similar impact in lush serpentine grassland that, in terms of soil nitrogen availability, is only slightly less fertile than nonserpentine grassland. Responses to watering alone in our nonserpentine grassland were partly contingent on the presence of certain fast-growing N-fixers (especially Vicia), which are less common on serpentine soils, potentially because of scarcity of cofactors, such as molybdenum (50). Our finding highlights that N-fixers can benefit greatly from rainfall addition, as has been found in other studies (5), and is consistent with the hypothesis of colimitation by water and N. Because N-fixers produce highly decomposable litter, such increases in their abundance as a result of enhanced precipitation can alter soil microbial communities, nutrient cycling, and even trophic interactions, with potentially remarkable ecosystem-level consequences (5, 51–53).
We found that the biomass increase in response to resource additions in the infertile grassland habitats was unconstrained by the resource-conservative functional strategies of the resident species in these habitats. We attribute this result, which contrasts with some previous studies (54), to the fine-grained spatial heterogeneity in our study system combined with the copious seed production of exotic annual grasses and tall forbs that dominate adjacent fertile grassland habitats. These factors likely ensured an abundant seed supply of tall and fast-growing annual grasses and forbs to our infertile grassland habitats, as evidenced by the fact that the watered and fertilized plots went through nearly complete species turnover and that it was these species rather than residents that dominated the biomass response. This result is consistent with previous studies that link cross-habitat movement of propagules to the maintenance of productivity and diversity (55–57), and responsiveness of communities to climate change (13, 58).
Our experiment also tested the role of functional traits as predictors of individual species responses to changed water availability. We found that species with low SLA and short stature, indicative of the resource-conservative syndrome (36–38), were more likely to decline under both water and nutrient additions, whereas species with the resource-acquisitive syndrome had higher probabilities of increasing. Functional traits have recently been invoked as potentially important determinants of the sensitivity of individual species to altered climatic conditions (59). In one recent study, species’ traits linked to conservative water use and belowground investment were associated with greater success under 20 y of observed climatic warming (60). Our finding that resource-conservative species were disfavored under enhanced water and nutrient supply suggest that slow growing/fast growing trait syndromes may predict which species will increase and which will decline under altered precipitation patterns and nutrient enrichment. Although our results are generally consistent with other studies that link high SLA and tall stature to more fertile soils and high soil moisture (26, 33, 42, 54, 61), our study is unique among these in that it uses traits as predictors of individual species’ vulnerability to global changes. Such losses of resource-conservative species are expected to exert positive feedbacks on whole-ecosystem functioning through the enhanced litter quality and faster nutrient cycling associated with the fast-growing functional trait syndrome (51, 52, 62). We conclude that traits provide a valuable species-independent currency for forecasting shifts in species distributions across ecosystems under altered rainfall and anthropogenic nutrient enrichment.
Our results demonstrate a mechanism by which low-productivity systems can exhibit greater resistance to climate change and show the predictive power that functional traits have in forecasting species’ distributional shifts. Our work thereby links the general theory of resource colimitation (17) and functional trait syndromes (36–38) to a substantial body of previous evidence from experiments (13, 14, 30, 32), analyses of natural climatic variability (26, 33) and geographic variability (63), and even results from paleoecology (31). Our findings illustrate the potential for ecological theory and experiments to improve our predictions of the ecological effects of climate change.
Materials and Methods
Study System.
Our study site lies at the University of California McLaughlin Reserve, in the Inner North Coast Range of California (N 38°52′, W 122°26′). The climate is Mediterranean, with mean annual temperatures of 8 °C in January and 25 °C in July, and mean annual rainfall of 62 cm, falling mainly in October–April. Our experimental site of roughly 1,000 m × 500 m is a complex of grasslands on varying soils, originating from variation in underlying bedrock material and soil depth. Substrates include infertile, nutrient-poor soils on shallow rocky slopes, derived from serpentine rocks, which support low-productivity, species-rich vegetation dominated by native annual forbs (“harsh serpentine grasslands”). Deeper and finer-textured serpentine soils on slopes and valley bottoms have higher nutrient concentrations and support more productive vegetation, consisting of a mixture of native and exotic species (“lush serpentine grasslands”). Other substrates include fertile, nutrient-rich soils, derived from sedimentary rocks, and supporting higher productivity and vegetation dominated by exotic (Eurasian) annual grasses and forbs (“nonserpentine grasslands”). At our experimental site these three grassland types are interspersed over relatively short distances (101–102 m), making it possible to replicate treatments on soils with different productivity levels and corresponding species compositional differences. When establishing experimental plots (see below), we first classified the habitats visually and then confirmed our classifications by detailed plant community and soil analyses (Table S1).
Rainfall and Nutrient Addition Experiment.
In 2010 we built an irrigation system consisting of nine watering lines that passed through multiple spatially interspersed patches of the three grassland habitats within a 12-ha area (Fig. S1). We established experimental 2 × 2-m plots (Fig. S2) along these lines and randomly allotted them to a full-factorial combination of rainfall addition and nutrient addition treatments. Several plots were later lost when heavy equipment drove over them, resulting in 10–12 replicates per treatment and habitat combination and 131 plots total. We based our watering treatment on previous work in Californian grasslands, which found water addition has strong effects in spring when rainfall has largely ceased and soils are drying rapidly, but little effect during the rainy winter season (5). Rainfall addition in spring corresponds to the predictions of some—though not all—climate forecasts for northern California (64, 65). After March 15 of each year, we began watering when rainfall had ceased for at least a week and none was forecast. Harvested rainwater was delivered through the watering lines to sprinkler heads that sprayed in a 3-m radius (Mini Rotor Drip Emitters, Olson Irrigation) placed 50 cm above the soil in the center of each 2 × 2-m plot (Fig. S2). We added 2.5 cm of water over a 12-h period at night to minimize evaporation, once a week for 8 consecutive weeks in each year (2010–2014). This mimicked a moderate storm event and increased total yearly precipitation by roughly 18% over the mean (5). From November 2010 to March 2014, we applied slow-release granular NPK (10-10-10) fertilizer with micronutrients (Lilly Miller Ultra Green) to our nutrient addition plots, using equal amounts in November, February, and March that totaled 10 g N/m−2, 10 g P/m−2, and 10 g K/m−2 per year (see www.nutnet.umn.edu/nutrients for a similar protocol used worldwide).
Community Measurements.
We visually estimated percent areal cover by each species in 1-m2 subplots. To account for species differences in phenology, we sampled in the early, middle, and late growing season (April, June, and August) and used the peak cover value for each species in each year. All vegetation surveys were done by the same trained and experienced person with a minimum estimate threshold of 0.1%. We used these data to calculate Simpson diversity, a standard metric that combines species richness and evenness. We also calculated Bray–Curtis dissimilarity of each plot in 2010 to the same plot in 2014 as a metric of compositional change. We collected aboveground biomass from subplots of 0.0625 m2 annually in late May to early June, when the total community productivity peaked, sorted it into live and litter components (where litter included both standing dead litter from the same year and litter remaining on the ground from the previous years), dried it for 3 d in +60 °C, and weighed it. To investigate which major plant groups were responsible for the biomass increase, we used the cover data to analyze the responses of all exotics (including exotic grasses), grasses, and N-fixers (Table S4). Previous studies have identified these particular groups as especially responsive to water and nutrient amendments (4, 5).
Table S4.
Functional groups and exotic/native status of the species found in the experimental plots in 2010–2014
| Species | Functional group | Exotic/native |
| Achyrachaena mollis | Forb | Native |
| Agoseris heterophylla | Forb | Native |
| Allium falcifolium | Forb | Native |
| Allium amplectens | Forb | Native |
| Amsinckia menziesii | Forb | Native |
| Anagallis arvensis | Forb | Exotic |
| Ancistrocarpus filagineus | Forb | Native |
| Aphanes occidentalis | Forb | Native |
| Astragalus breweri | N-fixer | Native |
| Astragalus gambelianus | N-fixer | Native |
| Athysanus pusillus | Forb | Native |
| Avena fatua | Grass | Exotic |
| Brodiaea elegans | Forb | Native |
| Bromus diandrus | Grass | Exotic |
| Bromus hordeaceus | Grass | Exotic |
| Bromus madritensis | Grass | Exotic |
| Calandrinia ciliata | Forb | Native |
| Calochortus luteus | Forb | Native |
| Calochortus superbus | Forb | Native |
| Calycadenia pauciflora | Forb | Native |
| Camissonia graciliflora | Forb | Native |
| Carduus pycnocephalus | Forb | Exotic |
| Castillea attenuata | Forb | Native |
| Castillea rubicundula | Forb | Native |
| Eriogonum nudum | Forb | Native |
| Eriogonum vimineum | Forb | Native |
| Eriophyllum lanatum | Forb | Native |
| Erodium brachycarpum | Forb | Exotic |
| Erodium cicutarium | Forb | Exotic |
| Euphorbia crenulata | Forb | Native |
| Filago californica | Forb | Native |
| Filago gallica | Forb | Native |
| Fritillaria purdyi | Forb | Native |
| Galium aparine | Forb | Native |
| Gastridium ventricosum | Grass | Exotic |
| Geranium dissectum | Forb | Exotic |
| Gilia capitata | Forb | Native |
| Gilia tricolor | Forb | Native |
| Hemizonia congesta | Forb | Native |
| Hesperolinon californicum | Forb | Native |
| Holocarpa virgata | Forb | Native |
| Hordeum brachyantherum | Grass | Native |
| Hypochaeris glabra | Forb | Native |
| Juncus bufonius | Grass | Native |
| Lactuca serriola | Forb | Exotic |
| Lagophylla minor | Forb | Native |
| Lasthenia californica | Forb | Native |
| Lepidium nitidum | Forb | Native |
| Lessingia ramulosa | Forb | Native |
| Linanthus bicolor | Forb | Native |
| Linanthus dichotomus | Forb | Native |
| Ranunculus californicus | Forb | Native |
| Ranunculus occidentalis | Forb | Native |
| Rigiopappus leptocladus | Forb | Native |
| Sagina californica | Forb | Native |
| Sagina apetala | Forb | Native |
| Sanicula sp. | Forb | Native |
| Senecio vulgaris | Forb | Exotic |
| Sidalcea diploscypha | Forb | Native |
| Silene gallica | Forb | Native |
| Sisyrinchium bellum | Forb | Native |
| Sonchus sp. | Forb | Exotic |
| Stellaria nitens | Forb | Native |
| Streptanthus breweri | Forb | Native |
| Taeniatherum caput-medusae | Grass | Exotic |
| Thysanocarpus curvipes | Forb | Native |
| Torilis arvensis | Forb | Exotic |
| Trifolium albopurpureum | N-fixer | Native |
| Trifolium bifidum | N-fixer | Native |
| Trifolium dubium | N-fixer | Exotic |
| Trifolium fucatum | N-fixer | Native |
| Trifolium gracilentum | N-fixer | Native |
| Trifolium hirtum | N-fixer | Exotic |
| Trifolium microcephalum | N-fixer | Native |
| Trifolium subterraneum | N-fixer | Exotic |
| Trifolium willdenovii | N-fixer | Native |
| Uropappus lindleyi | Forb | Native |
| Velezia rigida | Forb | Exotic |
| Centaurea solstitialis | Forb | Exotic |
| Centaurium trichanthum | Forb | Native |
| Cercocarpus betuloides | Shrub | Native |
| Cerastium glomeratum | Forb | Exotic |
| Chaenactis glabriuscula | Forb | Native |
| Chlorogalum pomeridianum | Forb | Native |
| Chorizanthe polygonoides | Forb | Native |
| Circium sp. | Forb | Exotic |
| Clarkia gracilis | Forb | Native |
| Clarkia purpurea | Forb | Native |
| Claytonia exigua | Forb | Native |
| Collinsia parsiflora | Forb | Native |
| Crassula connata | Forb | Native |
| Cryptantha hispidula | Forb | Native |
| Cryptantha micrantha | Forb | Native |
| Cuscuta californica | Forb | Native |
| Cynosurus echinatus | Grass | Exotic |
| Dactylis glomerata | Grass | Exotic |
| Daucus pusillus | Forb | Native |
| Delphinium variegatum | Forb | Native |
| Dichelostemma capitatum | Forb | Native |
| Elymus elymoides | Grass | Native |
| Epilobium brachycarpum | Forb | Native |
| Eremocarpus setigerus | Forb | Native |
| Linanthus jepsonii | Forb | Native |
| Lolium multiflorum | Grass | Exotic |
| Lomatium marginatum | Forb | Native |
| Lotus humistratus | N-fixer | Native |
| Lotus wrangelianus | N-fixer | Native |
| Lupinus bicolor | N-fixer | Native |
| Lupinus microcarpus | N-fixer | Native |
| Lupinus nanus | N-fixer | Native |
| Madia sp. | Forb | Native |
| Melica californica | Grass | Native |
| Medicago polymorpha | N-fixer | Exotic |
| Micropus californicus | Forb | Native |
| Microseris douglasii | Forb | Native |
| Mimulus douglasii | Forb | Native |
| Mimulus guttatus | Forb | Native |
| Minuartia californica | Forb | Native |
| Montia fontana | Forb | Native |
| Nassella pulchra | Grass | Native |
| Navarretia jepsonii | Forb | Native |
| Navarretia pubescens | Forb | Native |
| Nemophila pedunculata | Forb | Native |
| Petrorhagia prolifera | Forb | Exotic |
| Phlox gracilis | Forb | Native |
| Plantago erecta | Forb | Native |
| Plagiobothrys nothofulvus | Forb | Native |
| Poa secunda | Grass | Native |
| Polypogon monspeliensis | Grass | Exotic |
| Vicia villosa | N-fixer | Exotic |
| Viola douglasii | Forb | Native |
| Vulpia microstachys | Grass | Native |
| Vulpia myuros | Grass | Exotic |
| Zigadenus fremontii | Forb | Native |
Trait Data.
We measured SLA (leaf area in square millimeters per gram dry mass), foliar C:N (based on percent C and N in plant leaves), LWC, and plant height for all species occurring in the experimental plots. These traits are widely documented to have links with both water and nutrient balance, and relative growth rate (36–38, 43, 44). Trait data were collected in summer 2010 from 10 individuals per species in the study area following standard protocols (43). To represent the pretreatment functional composition of communities, each species’ relative abundance in 2010 was multiplied by that species’ trait values for each species and summed across species to give a community-weighted mean (CWM) value (66) for each trait in each plot.
Statistical Analyses.
To assess the impact of rainfall and nutrient additions on productivity in 2014, we used a LME model with community biomass (square root-transformed) as the response variable; habitat (grassland type), rainfall, and nutrient additions, and their interactions as explanatory variables; and watering line as a random variable. To assess treatment impacts on species diversity and compositional change, we used similar models with Simpson diversity, Bray–Curtis dissimilarity, and the cover of exotics, N-fixers, and grasses as the response variables. To examine the possible role of resident community traits in predicting the productivity response to the treatments, we applied similar LME models with community biomass as the response variable (square root-transformed), and the CWM trait values as covariates; each trait was examined in a separate model to avoid multicollinearity. To examine the role of plant traits in predicting individual species’ likelihood of either decline of increase in response to the treatments, we used generalized linear mixed effects (GLMM) models with a binomial error structure, in which the response variable was the probability of each species to decrease/increase from 2010 to 2014 in each plot, and the predictors were the treatments, the functional traits, and their interactions. Each trait was again tested in a separate model. We manually simplified the GLMM models to identify the most parsimonious models, and used a χ test to assess the significance of each explanatory variable (at the end, the models included only significant explanatory variables with P < 0.05).
We used the function “lme” in package nlme for LME, the function “diss” in package simba for Bray–Curtis dissimilarity between 2010 and 2014, and “glmer” in package lme4 for GLMM in R statistical software (67).
Acknowledgments
We thank Risto Virtanen, Scott Woodin, Ella Samuel, Gregory Wada, Krinzal Mathur, Annette Bieger, and Stella Copeland for field assistance; Howard Cornell, Anne Marie Panetta, Risto Virtanen, and Barbara Fernandez-Going for comments on the previous versions of the manuscript; and the University of California McLaughlin Reserve, Paul Aigner, Scott Moore, Leslie Scott, and Rhett Woerly for logistical support. Funding was provided by Academy of Finland Project 253385 and the Wihuri foundation (to A.E.); and National Science Foundation Grant DEB-1111716 (to S.P.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1508170112/-/DCSupplemental.
References
- 1.Intergovernmental Panel on Climate Change . In: The Regional Impacts of Climate Change: An Assessment of Vulnerability. Watson RT, Zinyowera MC, Moss RH, editors. Cambridge Univ Press; Cambridge, UK: 1997. [Google Scholar]
- 2.Intergovernmental Panel on Climate Change . In: Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation. Field CB, et al., editors. Cambridge Univ Press; Cambridge, UK: 2012. [Google Scholar]
- 3.Knapp AK, et al. Consequences of more extreme precipitation regimes for terrestrial ecosystems. Bioscience. 2008;58(9):811–821. [Google Scholar]
- 4.Zavaleta ES, et al. Grassland responses to three years of elevated temperature, CO2, precipitation, and N deposition. Ecol Monogr. 2003;73(4):585–604. [Google Scholar]
- 5.Suttle KB, Thomsen MA, Power ME. Species interactions reverse grassland responses to changing climate. Science. 2007;315(5812):640–642. doi: 10.1126/science.1136401. [DOI] [PubMed] [Google Scholar]
- 6.Wu Z, Dijkstra P, Koch GW, Peñuelas J, Hungate BA. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob Change Biol. 2011;17(2):927–942. [Google Scholar]
- 7.Cleland EE, et al. Sensitivity of grassland plant community composition to spatial vs. temporal variation in precipitation. Ecology. 2013;94(8):1687–1696. doi: 10.1890/12-1006.1. [DOI] [PubMed] [Google Scholar]
- 8.Kulmatiski A, Beard KH. Woody plant encroachment facilitated by increased precipitation intensity. Nat Clim Chang. 2013;3(9):833–837. [Google Scholar]
- 9.Sala OE, et al. Global biodiversity scenarios for the year 2100. Science. 2000;287(5459):1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- 10.Schröter D, et al. Ecosystem service supply and vulnerability to global change in Europe. Science. 2005;310(5752):1333–1337. doi: 10.1126/science.1115233. [DOI] [PubMed] [Google Scholar]
- 11.Loarie SR, et al. The velocity of climate change. Nature. 2009;462(7276):1052–1055. doi: 10.1038/nature08649. [DOI] [PubMed] [Google Scholar]
- 12.Thorne JH, Boynton RM, Flint LE, Flint AL. The magnitude and spatial patterns of historical and future hydrologic change in California’s watersheds. Ecosphere. 2015;6(2):24. [Google Scholar]
- 13.Grime JP, et al. Long-term resistance to simulated climate change in an infertile grassland. Proc Natl Acad Sci USA. 2008;105(29):10028–10032. doi: 10.1073/pnas.0711567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tielbörger K, et al. Middle-Eastern plant communities tolerate 9 years of drought in a multi-site climate manipulation experiment. Nat Commun. 2014;5:5102. doi: 10.1038/ncomms6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liebig J. Animal Chemistry, or Organic Chemistry in Its Application to Physiology and Pathology. Johnson Reprint Corporation; New York: 1842. [Google Scholar]
- 16.Danger M, Daufresne T, Lucas F, Pissard S, Lacroix G. Does Liebig’s Law of the Minimum scale up from species to communities? Oikos. 2008;117(11):1741–1751. [Google Scholar]
- 17.Harpole WS, et al. Nutrient co-limitation of primary producer communities. Ecol Lett. 2011;14(9):852–862. doi: 10.1111/j.1461-0248.2011.01651.x. [DOI] [PubMed] [Google Scholar]
- 18.Bloom AJ, Chapin FS, III, Mooney HA. Nutrient limitation in plants—An economic analogy. Annu Rev Ecol Syst. 1985;16:363–392. [Google Scholar]
- 19.Gleeson SK, Tilman D. Plant allocation and the multiple limitation hypothesis. Am Nat. 1992;139(6):1322–1343. [Google Scholar]
- 20.Elser JJ, et al. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett. 2007;10(12):1135–1142. doi: 10.1111/j.1461-0248.2007.01113.x. [DOI] [PubMed] [Google Scholar]
- 21.Fay PA, et al. Grassland productivity limited by multiple nutrients. Nature Plants. 2015;1:15080. doi: 10.1038/nplants.2015.80. [DOI] [PubMed] [Google Scholar]
- 22.Craine JM. Resource Strategies of Wild Plants. Princeton Univ Press; Princeton, NJ: 2009. [Google Scholar]
- 23.Harpole WS, Potts DL, Suding KN. Ecosystem responses to water and nitrogen amendment in a California grassland. Glob Change Biol. 2007;13(11):2341–2348. [Google Scholar]
- 24.Harpole WS, Tilman D. Grassland species loss resulting from reduced niche dimension. Nature. 2007;446(7137):791–793. doi: 10.1038/nature05684. [DOI] [PubMed] [Google Scholar]
- 25.Eskelinen A, Harrison S. Exotic plant invasions under enhanced rainfall are constrained by soil nutrients and competition. Ecology. 2014;95(3):682–692. doi: 10.1890/13-0288.1. [DOI] [PubMed] [Google Scholar]
- 26.Dwyer JM, Hobbs RJ, Wainwright CE, Mayfield MM. Climate moderates release from nutrient limitation in natural annual plant communities. Glob Ecol Biogeogr. 2015;24(5):549–561. [Google Scholar]
- 27.Schimel DS, et al. Climate and nitrogen controls on the geography and timescales of terrestrial biogeochemical cycling. Global Biogeochem Cycles. 1996;10(4):677–692. [Google Scholar]
- 28.Manzoni S, Schimel JP, Porporato A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology. 2012;93(4):930–938. doi: 10.1890/11-0026.1. [DOI] [PubMed] [Google Scholar]
- 29.Damschen EI, Harrison S, Arckerly DD, Fernandez-Going BM, Anacker BL. Endemic plant communities on species soils: Early victims or hardy survivors of climate change? J Ecol. 2012;100(5):1122–1130. [Google Scholar]
- 30.Grime JP, et al. The response of two contrasting limestone grasslands to simulated climate change. Science. 2000;289(5480):762–765. doi: 10.1126/science.289.5480.762. [DOI] [PubMed] [Google Scholar]
- 31.Briles CE, Whitlock C, Skinner CN, Mohr J. Holocene forest development and maintenance on different substrates in the Klamath Mountains, northern California, USA. Ecology. 2011;92(3):590–601. doi: 10.1890/09-1772.1. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez-Going B, Harrison S. Effects of experimental water addition depend on grassland community characteristics. Plant Ecol. 2013;214(5):777–786. [Google Scholar]
- 33.Fernandez-Going BM, Anacker BL, Harrison SP. Temporal variability in California grasslands: Soil type and species functional traits mediate response to precipitation. Ecology. 2012;93(9):2104–2114. doi: 10.1890/11-2003.1. [DOI] [PubMed] [Google Scholar]
- 34.Harrison SP, Gornish ES, Copeland S. Climate-driven diversity loss in a grassland community. Proc Natl Acad Sci USA. 2015;112(28):8672–8677. doi: 10.1073/pnas.1502074112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kruckeberg AR. Geology and Plant Life. Univ of Washington Press; Seattle, WA: 2005. [Google Scholar]
- 36.Reich PB, Walters MB, Ellsworth DS. From tropics to tundra: Global convergence in plant functioning. Proc Natl Acad Sci USA. 1997;94(25):13730–13734. doi: 10.1073/pnas.94.25.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright IJ, et al. The worldwide leaf economics spectrum. Nature. 2004;428(6985):821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 38.Reich PB. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J Ecol. 2014;102(2):275–301. [Google Scholar]
- 39.Rajakaruna N, Boyd RS, Harris TB, editors. Plant Ecology and Evolution in Harsh Environments. Nova Science Publishers; New York: 2014. [Google Scholar]
- 40.Huenneke LF, Hamburg SP, Koide R. Effects of soil resources on plant invasion and community structure in Californian serpentine grassland. Ecology. 1990;71(2):478–491. [Google Scholar]
- 41.Going BM, Hillerislambers J, Levine JM. Abiotic and biotic resistance to grass invasion in serpentine annual plant communities. Oecologia. 2009;159(4):839–847. doi: 10.1007/s00442-008-1264-y. [DOI] [PubMed] [Google Scholar]
- 42.Eskelinen A, Harrison S. Erosion of beta diversity under interacting global change impacts in a semi-arid grassland. J Ecol. 2015;103(2):397–407. [Google Scholar]
- 43.Pérez-Harguindeguy N, et al. New handbook for standardized measurement of plant functional traits worldwide. Aust J Bot. 2013;61(3):167–234. [Google Scholar]
- 44.Dwyer JM, Hobbs RJ, Mayfield MM. Specific leaf area responses to environmental gradients through space and time. Ecology. 2014;95(2):399–410. doi: 10.1890/13-0412.1. [DOI] [PubMed] [Google Scholar]
- 45.Mantyka-Pringle C, Martin TG, Rhodes JR. Interactions between climate and habitat loss effects on biodiversity: A systematic review and meta-analyses. Glob Change Biol. 2012;18(4):1239–1252. [Google Scholar]
- 46.Gottfried M, et al. Continent-wide response of mountain vegetation to climate change. Nat Clim Chang. 2012;2(2):111–115. [Google Scholar]
- 47.Collins SL, et al. Stability of tallgrass prairie during a 19-year increase in growing season precipitation. Funct Ecol. 2012;26:1450–1459. [Google Scholar]
- 48.Hudson JMG, Henry GHR. High arctic plant community resists 15 years of experimental warming. J Ecol. 2010;98(5):1035–1041. [Google Scholar]
- 49.Tilman D. Resource Competition and Community Structure. Princeton Univ Press; Princeton, NJ: 1982. [PubMed] [Google Scholar]
- 50.Walker RB. Molybdenum deficiency in serpentine barren soils. Science. 1948;108(2809):473–475. doi: 10.1126/science.108.2809.473. [DOI] [PubMed] [Google Scholar]
- 51.Cornwell WK, et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett. 2008;11(10):1065–1071. doi: 10.1111/j.1461-0248.2008.01219.x. [DOI] [PubMed] [Google Scholar]
- 52.de Vries FT, et al. Abiotic drivers and plant traits explain landscape-scale patterns in soil microbial communities. Ecol Lett. 2012;15(11):1230–1239. doi: 10.1111/j.1461-0248.2012.01844.x. [DOI] [PubMed] [Google Scholar]
- 53.Bardgett RD, Wardle DA. Aboveground-Belowground Linkages: Biotic Interactions, Ecosystem Processes and Global Change. Oxford Univ Press; Oxford, UK: 2010. [Google Scholar]
- 54.Eskelinen A, Harrison S, Tuomi M. Plant traits mediate consumer and nutrient control on plant community productivity and diversity. Ecology. 2012;93(12):2705–2718. doi: 10.1890/12-0393.1. [DOI] [PubMed] [Google Scholar]
- 55.Tilman D. Community invasibility, recruitment limitation, and grassland biodiversity. Ecology. 1997;78(1):81–92. [Google Scholar]
- 56.Stein C, Auge H, Fischer M, Weisser WW, Prati D. Dispersal and seed limitation affect diversity and productivity of montane grasslands. Oikos. 2008;117(10):1469–1478. [Google Scholar]
- 57.Myers JA, Harms KE. Seed arrival, ecological filters, and plant species richness: A meta-analysis. Ecol Lett. 2009;12(11):1250–1260. doi: 10.1111/j.1461-0248.2009.01373.x. [DOI] [PubMed] [Google Scholar]
- 58.Moser B, Friedley JD, Askew AP, Grime JP. Simulated migration in a long-term climate change experiment: Invasions impeded by dispersal limitation, not biotic resistance. J Ecol. 2011;99(5):1229–1236. [Google Scholar]
- 59.McMahon SM, et al. Improving assessment and modelling of climate change impacts on global terrestrial biodiversity. Trends Ecol Evol. 2011;26(5):249–259. doi: 10.1016/j.tree.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 60.Soudzilovskaia NA, et al. Functional traits predict relationship between plant abundance dynamic and long-term climate warming. Proc Natl Acad Sci USA. 2013;110(45):18180–18184. doi: 10.1073/pnas.1310700110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suding KN, et al. Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc Natl Acad Sci USA. 2005;102(12):4387–4392. doi: 10.1073/pnas.0408648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baxendale C, Orwin KH, Poly F, Pommier T, Bardgett RD. Are plant-soil feedback responses explained by plant traits? New Phytol. 2014;204(2):408–423. doi: 10.1111/nph.12915. [DOI] [PubMed] [Google Scholar]
- 63.Fernandez-Going BM, Harrison SP, Anacker BL, Safford HD. Climate interacts with soil to produce beta diversity in Californian plant communities. Ecology. 2013;94(9):2007–2018. doi: 10.1890/12-2011.1. [DOI] [PubMed] [Google Scholar]
- 64.National Assessment Synthesis Team . Climate Change Impacts on the United States: The Potential Consequences of Climate Variability and Change. US Global Change Research Program; Washington, DC: 2000. [Google Scholar]
- 65.Cayan D, Tyee M, Pierce D, Das T. 2012. Climate Change and Sea Level Rise Scenarios for California Vulnerability and Adaptation Assessment (California Energy Commission, Sacramento, CA), Publ No CEC-500-2012-008. Available at uc-ciee.org/climate-change/california-vulnerability-and-adaptation-study.
- 66.Garnier E, et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology. 2004;85(9):2630–2637. [Google Scholar]
- 67.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2014. Version 3.1.2. Available at www.R-project.org. [Google Scholar]