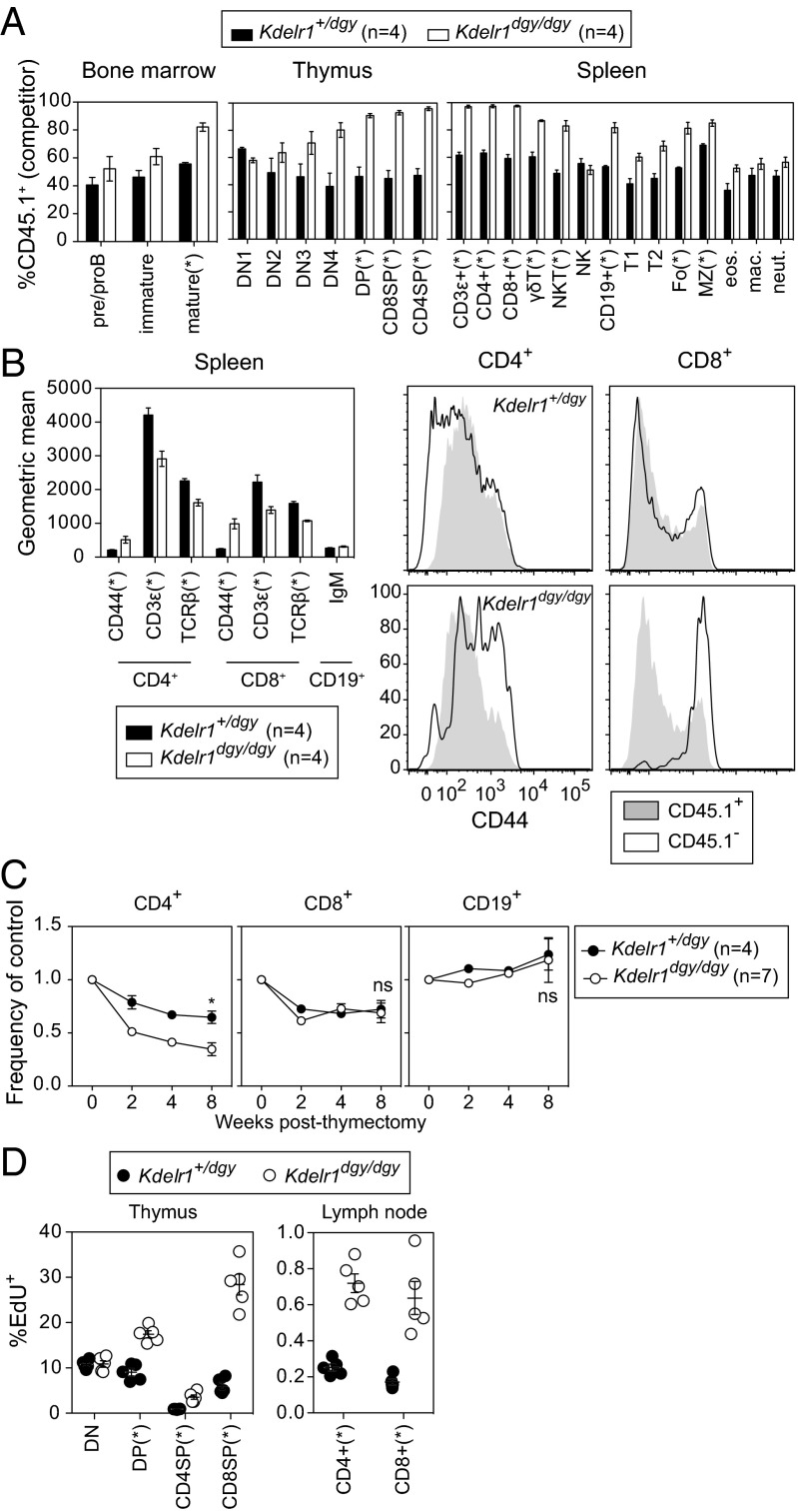
Fig. 6.
Cell-intrinsic defects in lymphocyte development, surface marker expression, survival, and proliferation. (A) Lethally irradiated Rag1 mutant recipients (CD45.2+) were transplanted with an equal mixture of wild-type (CD45.1+) and heterozygous or homozygous Kdelr1 mutant (CD45.2+) bone marrow. Wild-type donor chimerism (CD45.1+) was measured 8 wk later in the spleen, bone marrow, and thymus. Bone marrow subsets were gated as follows: preproB (B220+IgM−), immature (B220intIgM+), mature (B220hiIgM+). Thymic subsets: ETP (Lin−CD44+CD25−CD117+), DN2 (Lin−CD44+CD25+), DN3 (Lin−CD44−CD25+), DN4 (Lin−CD44−CD25−), DP (CD4+CD8α+), CD4SP (CD4+CD8α−), and CD8SP (CD4−CD8α+). Lineage markers were CD11b, CD3ε, B220, Ter119, Ly6G, NK1.1, and CD8α. Spleen subsets: T1, T2, Fo, and MZ B cells were gated as in Fig. 5. γδT (CD3ε+γδTCR+), NKT (NK1.1+CD3ε+), NK (NK1.1+CD3ε−), B (CD19+), eosinophils (Lin−CD11b+F4/80+SSChi), macrophages (Lin−CD11b+F4/80+SSClo), and neutrophils (Lin−CD11b+F4/80−Ly6Ghi). Lineage markers included CD19, TCRβ, NK1.1, and the viability dye 7-AAD. (B) Geometric mean surface expression of CD44, CD3ε, and TCRβ on CD4+ and CD8+ cells in mixed bone marrow chimeras. Surface IgM was measured on CD19+ cells. Histogram overlays show relative expression of CD44 on CD4+ and CD8+ cells. (C) Thymectomized mice were bled at the indicated weeks after surgery, and frequencies of CD4+, CD8+, and CD19+ cells were normalized to the average value in nonthymectomized littermates (set as 1). (D) Mice were injected with the thymidine analog EdU 4 h before organ harvest, and the percentages of EdU+ cells within various T-cell subsets was quantified. Asterisks represent P values less than 0.05 as determined by unpaired t tests (only performed at week 8 time point in C). Each symbol in D represents an individual mouse. Data are representative of one (C and D) or two (A and B) experiments, with at least four mice per genotype (error bars represent SEM).