Figure 1.
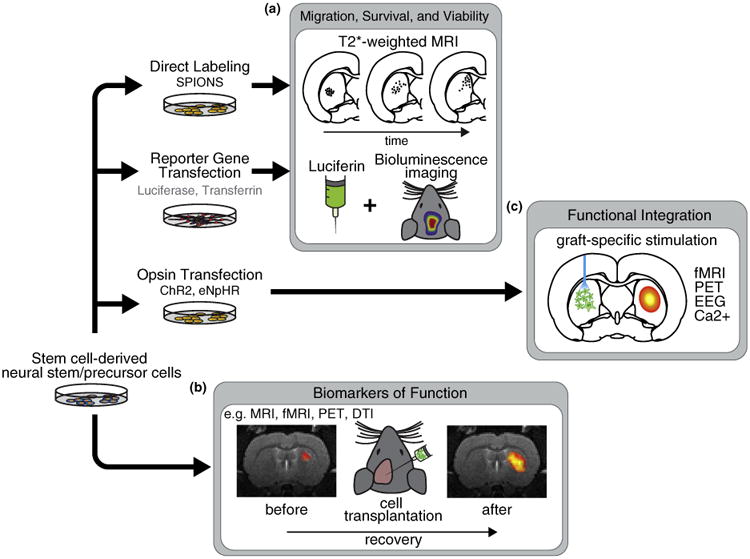
Imaging cell-based therapies for neurological disorders. (a) Cell migration and viability can be assessed by direct labeling, for example, with super paramagnetic iron oxide nanoparticles (SPIONs), or the insertion of reporter genes, for example, luciferase for bioluminescence. (b) In the long-term, imaging can be used as a biomarker of functional improvement. For example, functional magnetic resonance imaging (fMRI) can be combined with electrical, pharmacological or task-related stimuli. PET has been applied to assess the expression of specific receptors or transporters. Diffusion tensor imaging (DTI) has recently been used to assess myelin formation (see section on ‘Biomarkers of function’). (c) Cells can be modified to express light sensitive opsins such as channelrhodopsin-2 (ChR2) or halorhodopsin (eNpHR). Cells are then differentiated and transplanted to the brain. Over time, cells may become integrated with host circuitry and form functional synapses with remote regions. In combination with simultaneous functional imaging or EEG, different stimulation paradigms could be used to interrogate the specific dynamics of newly formed circuits.
