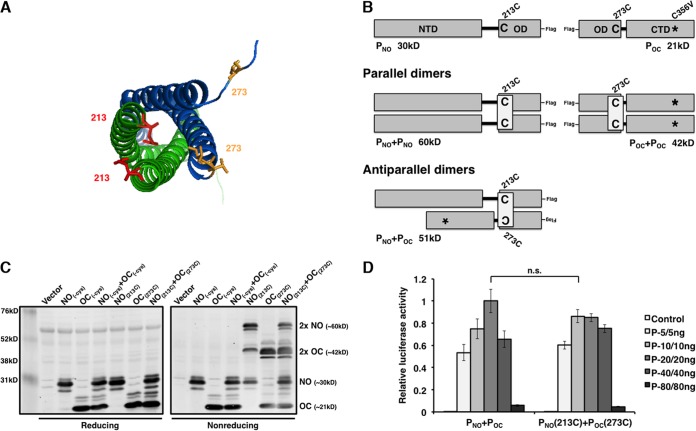
FIG 4.
P mutants with engineered cysteine residues dimerize in parallel orientation with biological activity. (A) Pairs of parallel chains are shown in green or blue. Side chains of mutated amino acids 213 (red) and 273 (yellow) are highlighted. The amino acid 213 residue not contained in the file was superimposed from the adjacent parallel chain. The figure was generated using the PyMOL program (43). (B) Schematic representation of incorporated cysteine residues for disulfide bond engineering. The sizes of the dimers in the parallel and antiparallel orientations are provided. (C) Assessment of the parallel oligomerization status of P deletion mutants containing cysteine residues under reducing and nonreducing conditions. (D) Minigenome activity of engineered P deletion mutants. Increasing amounts of P mutants were transfected with other plasmids as described in Materials and Methods. P values were calculated using Student's t test. Error bars represent the SEMs of data from six replicates. n.s., not significant.