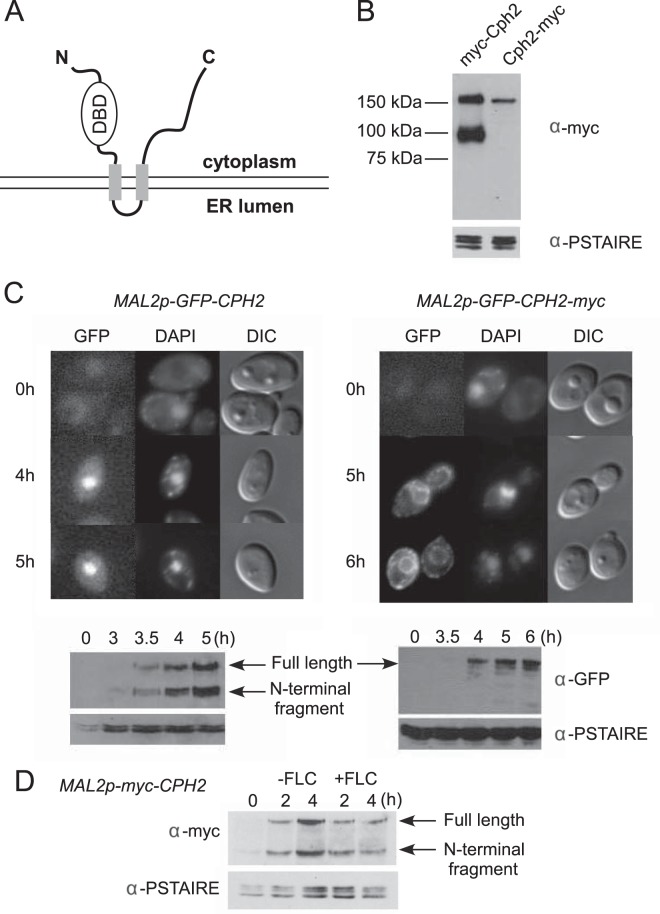
FIG 1.
Processing and nuclear localization of the Cph2 N-terminal domain from a transmembrane Cph2 protein. (A) Predicted configuration of Cph2 across a membrane. Cph2 has two predicted transmembrane segments (aa 408 to 430 and 501 to 523), with the N terminus and C terminus facing the cytosol. The N terminus contains a DNA binding domain similar to mammalian SREBPs and S. pombe Sre1. (B) Western blot of N- and C-terminally tagged Cph2. Cells of both Cph2p-myc-Cph2 (HLY3927) and Cph2p-Cph2-myc (HLY3829) strains were inoculated into fresh YPD (1:100) at 30°C and grown for 4 h for protein extraction. (C) Cellular localization of GFP-tagged Cph2 and Cph2-myc. Cells carrying Mal2p-GFP-Cph2 (HLY3918) and Mal2p-GFP-Cph2-myc (HLY3919) from overnight in SCD were inoculated into SC–2% maltose. After the indicated hours of growth, cells were collected for fluorescence microscopy and Western analysis. Cells with representative GFP localizations are shown. The C-terminal myc tag seems to have slowed down Cph2 processing and the transition of GFP-Cph2 from the ER to the nucleus. (D) Cph2 processing is not affected by the addition of fluconazole. Wild-type cells containing Mal2p-myc-Cph2 (HLY3968) were grown in YPD overnight at 30°C. Cells were washed 3 times with water, inoculated into YPM at 30°C with or without 10 μM fluconazole (FLC), and collected after 2 and 4 h for Western analysis.