Abstract
Gynecomastia refers to the enlargement of the male breast due to a proliferation of ductal, stromal, and/or fatty tissue. Although it is a common condition affecting up to 65% of men, not all cases require surgical intervention. Contemporary surgical techniques in the treatment of gynecomastia have become increasingly less invasive with the advent of liposuction and its variants, including power-assisted and ultrasound-assisted liposuction. These techniques, however, have been largely limited in their inability to address significant skin excess and ptosis. For mild to moderate gynecomastia, newer techniques using arthroscopic morcellation and endoscopic techniques promise to address the fibrous component, while minimizing scar burden by utilizing liposuction incisions. Nevertheless, direct excision through periareolar incisions remains a mainstay in treatment algorithms for its simplicity and avoidance of additional instrumentation. This is particularly true for more severe cases of gynecomastia requiring skin resection. In the most severe cases with significant skin redundancy and ptosis, breast amputation with free nipple grafting remains an effective option. Surgical treatment should be individualized to each patient, combining techniques to provide adequate resection and optimize aesthetic results.
Keywords: gynecomastia, surgical treatment of gynecomastia, gynecomastia review, gynecomastia techniques
Gynecomastia refers to any enlargement of the male breast due to a proliferation of ductal, stromal, and/or fatty tissue. Transient enlargement is often seen in the infant or adolescent patient as a part of normal development, and in most patients, a period of observation is appropriate. In patients outside of the typical pubertal or senescent age periods, a higher suspicion must be maintained for pharmacological etiologies, such as spironolactone, ketoconazole, calcium channel blockers, or marijuana, and pathological etiologies, including cirrhosis, adrenal/testicular neoplasms, or hypogonadism. The initial workup of the newly presenting patient has been well described. Often, the consulting surgeon is best served enlisting the expertise of an endocrinologist before proceeding with surgical correction.1
The treatment of gynecomastia has evolved toward less invasive approaches. With the advent of suction-assisted and ultrasound-assisted lipectomy, the majority of gynecomastia patients can achieve excellent results with minimal scar burden. A select group of patients with significant amounts of excess skin and ptosis, however, may still require a more invasive approach with the associated scarring seen in traditional reduction mammoplasty. Many modifications have been described in recent years to improve on the results of these standard techniques. These procedures and their indications are discussed as well as the preferences of the senior authors based on the severity of gynecomastia.
Classification
Although various classification schemes have been proposed, those most often cited are those set forth by Simon and Rohrich (Tables 1, 2).1 2 Letterman and Schurter proposed an early system based on operative requirements including skin excision and nipple repositioning.3 Simon moved away from this treatment-defined classification toward a qualitative classification of volume and skin redundancy dictating treatment. Attempting to improve on the subjectivity of Simon's initial classification system, Rohrich's classification includes estimates of total tissue mass requiring excision and further subdivides patients into those with adipose versus fibrous tissue predominance. A more recently published system from Monarca et al further expands on Rohrich's classification to include consideration of the overall chest shape and the presence of a sternal notch, helping to determine treatment considerations including “chest virilization” through development of a trapezoidal chest and emphasis of medial sternal muscular insertions.4
Table 1. Simon classification.
| Grade 1 | Small enlargement, no skin excess |
| Grade 2a | Moderate enlargement, no skin excess |
| Grade 2b | Moderate enlargement with extra skin |
| Grade 3 | Marked enlargement with extra skin |
Source: Adapted from Simon BE, Hoffman S, Kahn S. Classification and surgical correction of gynecomastia. Plast Reconstr Surg 1973;51:48.
Table 2. Rohrich classification.
| Grade I | Minimal hypertrophy (< 250 g of breast tissue) without ptosis |
| IA | Minimal hypertrophy—primarily glandular |
| IB | Minimal hypertrophy—primarily fibrous |
| Grade II | Moderate hypertrophy (250–500 g of breast tissue) without ptosis |
| IIA | Moderate hypertrophy—primarily glandular |
| IIB | Moderate hypertrophy—primarily fibrous |
| Grade III | Severe hypertrophy (> 500 g of breast tissue) with grade I ptosis (glandular or fibrous) |
| Grade IV | Severe hypertrophy with grade II or III ptosis (glandular or fibrous) |
Source: Adapted from Rohrich RJ, Ha RY, Kenkel JM, et al. Classification and management of gynecomastia: defining the role of ultrasound-assisted liposuction. Plast Reconstr Surg 2003;111:909.
Treatment
Prior to considering any surgical interventions, a medical evaluation should be performed, and withdrawal of offending pharmacological agents, tumor extirpation, and correction of any underlying systemic disease processes should be completed first. Any suspicion for an existing malignant breast tumor must be addressed prior to definitive therapy. Contemporary surgical options currently focus on initial liposuction for the removal of excess fatty tissue. Alternative modalities are utilized for removing any residual glandular tissue and/or excess skin.
Mild to Moderate Gynecomastia (Simon Grades I and IIa, and Rohrich Grades I and II)
Milder forms of gynecomastia are quite common and though 65% of men are thought to have some degree of gynecomastia, the proportion seeking surgical treatment is much lower. Thus, milder forms of gynecomastia may be underrepresented in the surgical population. Patients with minimal glandular hypertrophy typically have little skin excess and are treated readily with liposuction, frequently as the definitive treatment (Fig. 1). Some patients present with small amounts of fibrous gynecomastia well localized under the nipple and may be effectively treated with direct excision using a small periareolar incision (Fig. 2).
Fig. 1.
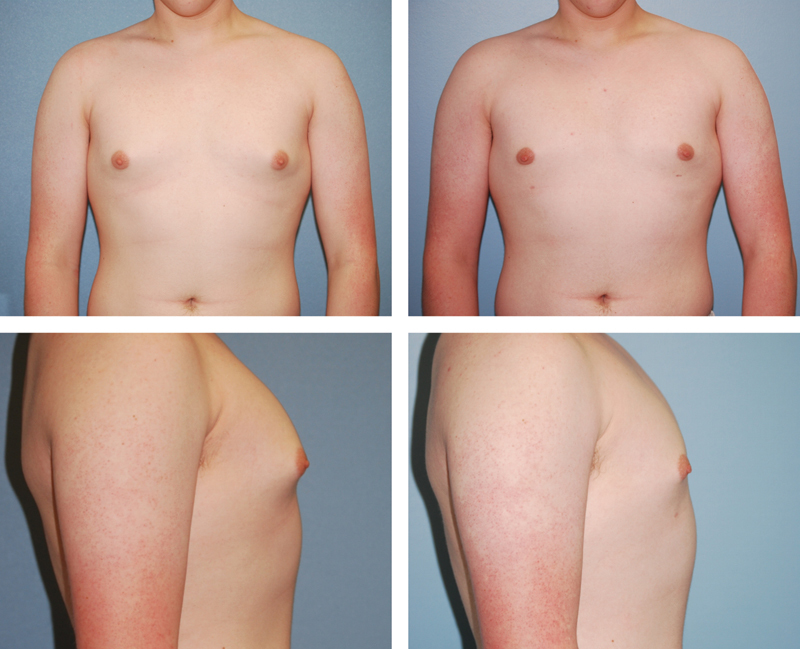
A 34-year-old man with mild gynecomastia treated with vibration amplification of sound energy at resonance (VASER) liposuction alone, shown preoperatively on the left and 7 months postoperatively on the right.
Fig. 2.
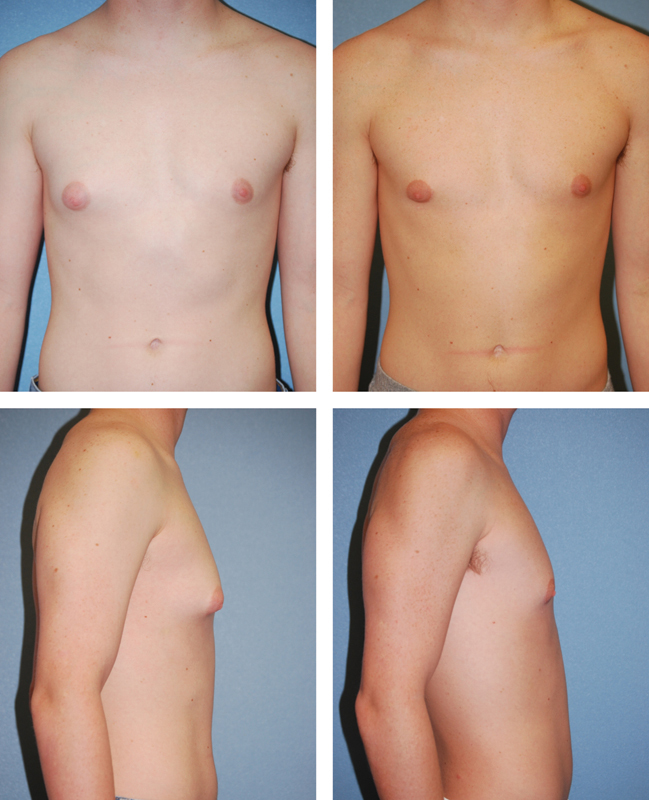
An 18-year-old man with mild gynecomastia treated with direct excision alone, shown preoperatively on the left and 5 months postoperatively on the right.
Refinements in the management of gynecomastia with liposuction have progressed with the introduction of new liposuction technologies. Power-assisted (PAL) and ultrasound-assisted liposuction (UAL) technologies have increased the extent of tissue removal capable by liposuction alone.5 Power-assisted liposuction has been described to reduce operative fatigue and increase control in chest wall contouring.6 7 Ultrasound-assisted liposuction has been well described by several authors as a primary, and often sole treatment modality for gynecomastia correction.1 8 9 The improved skin retraction often associated with UAL has allowed it to be used as a definitive treatment modality in many cases. Relatively high energy levels are used, with higher levels focused under the nipple to assist with removing the fibrous glandular tissue. Access ports are generally made at the lateral inframammary fold (IMF) combined with a periareolar or upper anterior axillary incision to allow for cross-hatching. Unlike traditional liposuction, the immediate subdermal layer is targeted with the goal of increased skin contraction, but this must be done with care due to the risk of thermal injury. Vibration amplification of sound energy at resonance- (VASER; Sound Surgical Technologies, LLC, Louisville, CO) assisted liposuction, is a newer form of UAL technology utilizing the application of alternating ultrasonic energy, which is considered by many practitioners to be a safer modality when treating fibrous areas close to the skin surface.10 If a prominent IMF is present, it should be aggressively undermined and disrupted to allow for re-draping and contraction of the skin in this area. The more adherent upper lateral pectoral region, where fullness is better tolerated, should not be overtreated. Postoperatively, patients are placed in compression garments for 6 to 8 weeks and foam dressings are frequently used in the immediate postoperative period. Authors advocating this technique report better extraction of fibrous fat, better contour control, and increased skin contraction postoperatively, often obviating, or substantially reducing the need for future skin resection. In a head-to-head comparison by Wong et al, UAL required fewer conversions to open excision and a lower rate of revisions for skin excision.11
Even in mild forms of gynecomastia, there may be a residual glandular component that needs to be addressed after liposuction. A low threshold for direct excision should be maintained, as residual firm, subareolar glandular tissue can be a great source of patient dissatisfaction.12 In patients for whom liposuction is not entirely sufficient, glandular tissue requires direct excision either primarily, or in a staged fashion, accompanying skin excision. Direct excision techniques through transareolar and periareolar incisions were first described by Webster in 1946 and remain a mainstay of treatment.2 3 13 However, with the increasing use of liposuction as initial therapy, many newer techniques for glandular excision have been developed with the goal of utilizing the liposuction incisions and minimizing the need for additional scars.
Early techniques utilizing the liposuction incisions include a “pull through” technique described by Morselli, which describes blindly dissecting the breast parenchymal tissue from the skin and pectoral fascia then grasping and pulling the tissue out through the liposuction incision for piecemeal excision.14 15 16 17 Various devices, such as reinforced or laser-sharpened cannulas, have been explored to allow for removal of this fibrous component as well.18 19
Most recently, the use of orthopedic arthroscopic shavers has gained popularity. In a series reported by Prado and Castillo in 2005, patients were treated with liposuction followed by arthroscopic shaver morcellation to address any residual glandular component.20 As described, bilateral 5-mm inframammary incisions are used to insert liposuction cannulas followed by the arthroscopic shavers. The arthroscopic shaver cannulas have dentated double lumen tips that rotate at 4000 to 6000 rpm to mechanically separate fibrous glandular tissue for suctioning. This technique is useful for mild to moderate gynecomastia not requiring skin excision and takes advantage of orthopedic instruments readily available in many hospitals.
Those desiring the control of visualization with minimal incisions can also consider subcutaneous endoscopic techniques, described by Ohyama et al.21 Although endoscopic surgery typically requires a minimum of three incisions for the camera and instrumentation, these techniques have also become increasingly less invasive with Jarrar et al in 2011 describing liposuction coupled with piecemeal excision under endoscopic visualization from a single 15-mm lateral chest-wall incision hidden in the axilla.22
Despite the introduction of these new techniques, direct excision through a periareolar incision remains popular due to its simplicity and use of standard instrumentation. In patients with any concern for increased risk of malignancy, such as with Klinefelter syndrome, no liposuction should be done, and only direct excision should be performed to allow for pathologic evaluation. Commonly, incisions are made slightly within the areolar margin along the inferior half of the circumference of the areola for an inconspicuous scar. From this incision, direct excision of small areas of residual fibrous glandular tissue or a complete subcutaneous mastectomy can be performed, carefully leaving sufficient subareolar glandular tissue to avoid a depression or saucer deformity.12
Severe Gynecomastia (Simon Grade IIB and Rohrich Grade III)
Patients with severe gynecomastia will usually require some form of skin resection. With the significant increase in patients undergoing bariatric weight loss surgery, more and more of these male patients with significant excess skin are presenting for reduction. Many techniques utilizing various skin excision patterns and pedicles similar to those used in female mastopexy and reduction mammoplasty have been used. In the 1970s, Letterman described the use of an oblique Dufourmentel-Mouly procedure based on an elliptical incision with a bipedicled dermal areolar flap.23 The consequence, however, was a large oblique extra-areolar scar extending laterally, much like a traditional mastectomy. Other techniques have described using Wise-pattern scars and glandular pedicles similar to those in traditional reduction mammoplasty. These techniques present many drawbacks for male patients. Not only do these procedures often leave excess glandular tissue behind, but the Wise pattern frequently causes coning of the breast and unacceptable scarring. Huang et al recognized these issues and in 1982 described a series of patients treated with a circumareolar excision to allow for skin excision without extra-areolar scarring.24 This technique relies on a central mound with an intercostal blood supply through the prepectoral fascia. Similar techniques, as described by Botta, recognize nipple–areolar complex (NAC) viability on the subdermal plexus alone and utilize superiorly based dermo-glandular flaps, allowing for a more uniform excision of breast tissue (Fig. 3).25 26 The periareolar approach has become the favored technique of the senior authors, taking advantage of its inconspicuous scar and flattening of the breast. This technique can be performed at the primary operation in conjunction with liposuction and/or direct excision to achieve a single stage correction, or it can be done in a second stage for skin excision alone in patients with inadequate skin contraction after UAL.
Fig. 3.
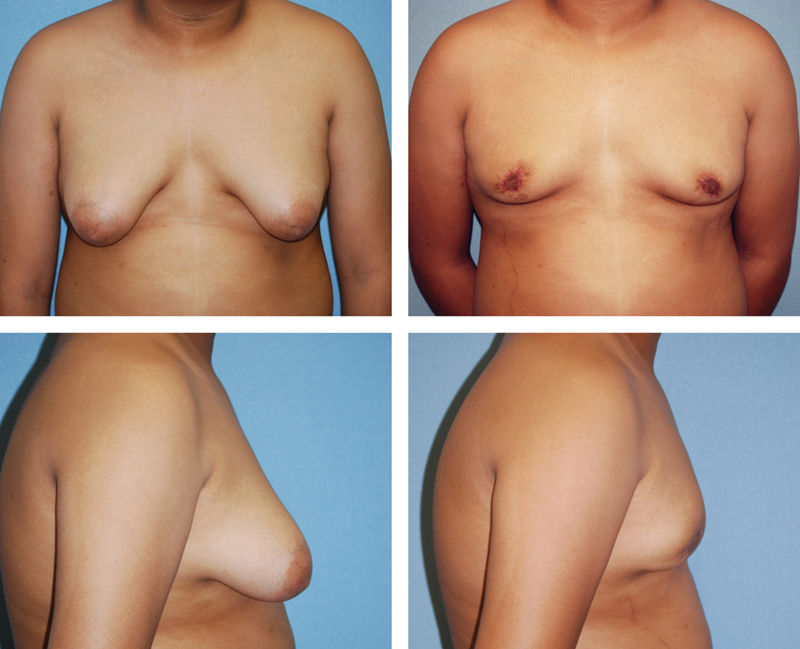
A 13-year-old boy with severe gynecomastia and ptosis treated with a periareolar skin excision on a superiorly based dermoglandular pedicle with direct excision of glandular tissue, shown preoperatively on the left and 4 months postoperatively on the right.
With the patient standing, the mid-clavicular line, IMF, and the intended areas of suctioning are marked preoperatively. Once in the operating room, an ∼3 cm wide oval NAC is marked along with the planned area of skin excision guided by a pinch test. Care should be taken in designing the extent of skin and areolar excision to prevent closing the wounds under excessive tension, which can worsen scarring and cause nipple distortion (Fig. 4). Liposuction of the deep planes is performed first, with care to avoid disrupting the subdermal plexus. Next, the skin between the new areolar marking and the planned periareolar excision is de-epithelialized. A transdermal incision can then be made at the inferior border of the de-epithelialized region to allow for direct glandular excision, if needed, carefully leaving a sufficiently thick superior dermoglandular NAC flap to maintain the viability of the NAC and avoid a saucer deformity (Fig. 5). After the excision is complete, a drain is placed and the transdermal incision is closed with absorbable suture. The periareolar incision is then closed in layers, taking care to distribute the excess skin evenly around the areola.26
Fig. 4.
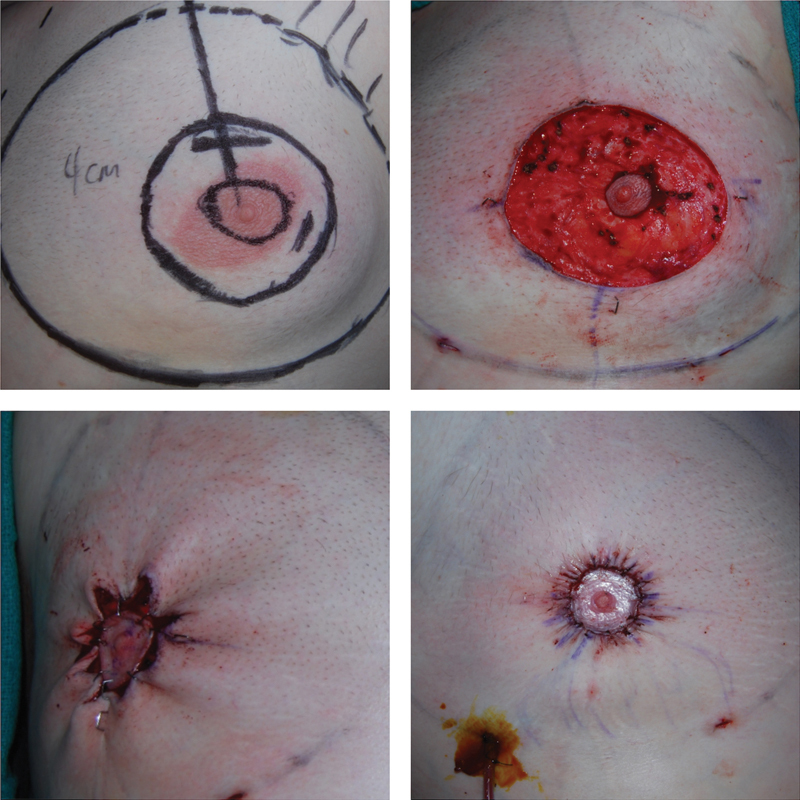
The top left image shows preoperative markings of the new nipple–areolar complex (NAC) size and planned periareolar skin incision. The top right image shows an intraoperative photo of the dermoglandular pedicle and de-epithelialization of the periareolar skin. The bottom images show a pursestring closure of the remaining skin to the new NAC with care to avoid significant nipple distortion.
Fig. 5.
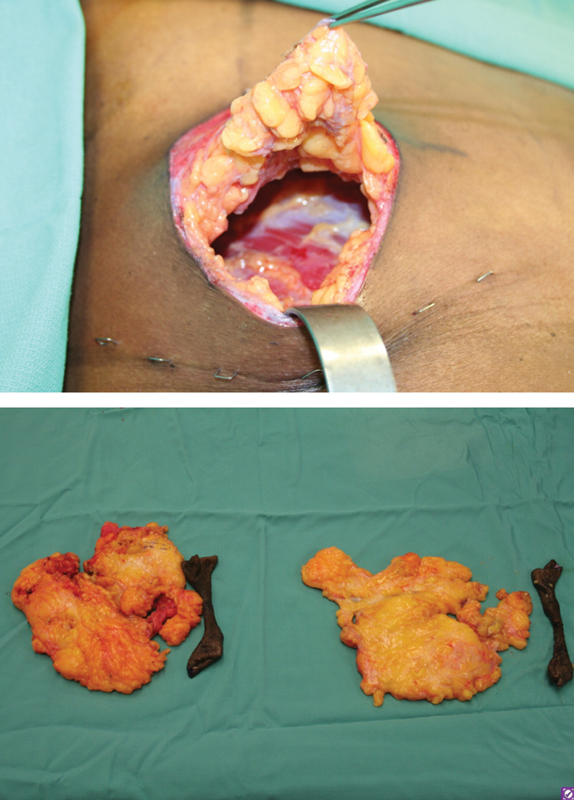
The top image shows an intraoperative photo of the patient in Figure 3, demonstrating an inferior transdermal incision to allow for direct excision of tissue after a periareolar skin excision. The nipple–areolar complex is preserved on a superiorly based dermoglandular pedicle. The bottom image shows the excised skin and breast tissue from the same patient.
In female mammaplasty, the circumareolar approach has been criticized for causing unfavorable flattening of the breast and an oval shape of the nipple. These sequelae serve as benefits in gynecomastia patients, as this is the desired effect. In contrast, the traditional Wise-pattern reduction has the opposite effect of coning the breast and should be avoided in male patients. The periareolar approach has limitations in terms of the degree of resection, and attempts at excessive skin excision can lead to widened scars, nipple distortion, and periareolar corrugation/rippling. Although these effects are not ideal, many times they are less noticeable and more easily accepted by patients than the burden of a long, extra-areolar scar crossing the entire chest or the hypopigmentation that can be seen with free nipple grafting. Widened scars and rippling can also be addressed with a minor revision in the office if needed. In the senior surgeons' experience, the periareolar pattern should be used in almost all patients who require skin resection except for the most extreme cases.
Severe Gynecomastia with Grade II or III Ptosis (Simon Grade III and Rohrich Grade IV)
In cases of severe gynecomastia with marked ptosis and extreme skin excess, other incisions which cause extra-areolar scarring may be required. For these cases, the most reliable and simple technique is breast amputation using an IMF incision with free nipple grafting (Fig. 6).25 These procedures have become increasingly necessary as the population of massive-weight-loss patients grows.
Fig. 6.
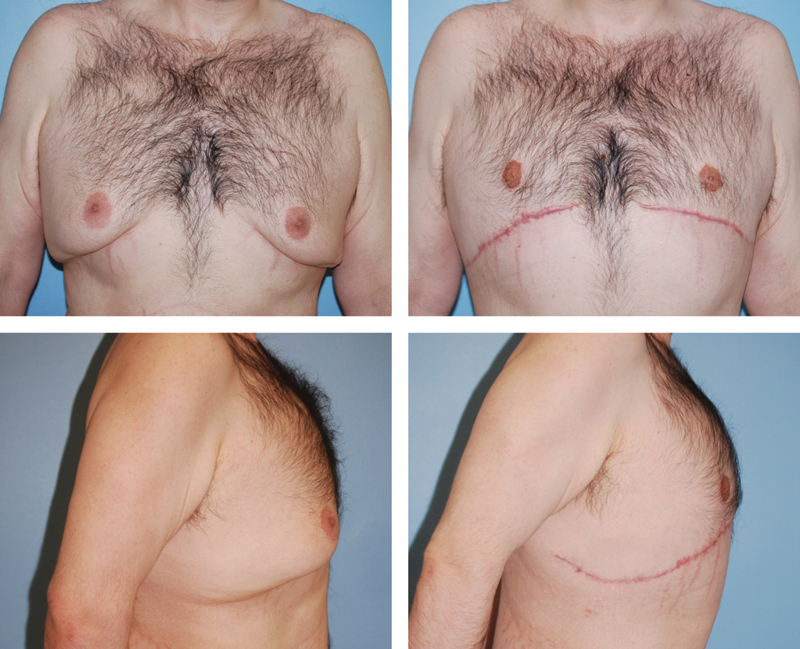
A 28-year-old man with severe gynecomastia and ptosis after massive weight loss treated with breast amputation and free nipple graft, shown preoperatively on the left and 3 months postoperatively on the right.
As with previous methods, the IMF and new nipple position are marked. The new NAC is drawn as a horizontal oval ∼3 cm in diameter at the fourth intercostal space; however, the size varies depending on the patient's overall body habitus. Again, initial liposuction is performed. The incision is made in the IMF and carried down to the level of the pectoral fascia. The glandular tissue is then dissected off the fascia to the level of the second intercostal space. The nipple is removed as a full-thickness graft. The superior flap is then pulled inferiorly to estimate and mark the excision of excess skin and soft tissue. The IMF incision is closed in layers over a drain. Finally, the nipple is placed onto a de-epithelialized bed and secured with a bolster dressing. The location of the NAC can be estimated at the fourth intercostal space in the midclavicular line; however, the patient should be viewed in the upright position on the operating table to ensure appropriate position for each patient depending on their body habitus.
For patients unwilling to accept loss of nipple sensation or possible depigmentation seen with free nipple graft techniques, elliptical excision patterns allow for significant skin excision while still maintaining NAC viability on a pedicle, and may be a viable alternative to amputation and free nipple grafting.23 The transverse elliptical excision of skin and soft tissue has been described for Simon grade III gynecomastia, and results in a horizontal scar extending medially and laterally off the areola.27 This pattern has been applied to various dermo-glandular pedicles and is well suited to the male chest while minimizing nipple distortion.28 The issue of severe ptosis is also frequently encountered in massive-weight-loss patients presenting with pseudogynecomastia, defined as skin excess with the proliferation of breast adipose tissue without glandular enlargement. The use of the transverse elliptical incision on a superolateral pedicle has been described as an alternative to amputation and free nipple grafting in this population.29 All of these techniques involve a scar that crosses the midchest passing around the areola. In our opinion, the IMF scar is less conspicuous and better accepted. In addition, the need to maintain a glandular pedicle in any form of male reduction mammaplasty can lead to excess remaining tissue, leading to a contour deformity and undercorrection.
Complications/Outcomes
Potential complications from gynecomastia surgery include hematoma, seroma, infection, inadequate resection, poor scarring, contour deformity, breast asymmetry, sensory changes, and pain. Given differences in documentation, the overall rate of complications is difficult to assess. Nevertheless, the overall complication rate has been reported between 14.5 to 53%, with hematoma being the most common.30 31 32 In mild gynecomastia treated with liposuction alone or with an arthroscopic shaver, reported hematoma rates are as low as 1%, whereas open subcutaneous mastectomies show a higher rate between 11 to 16%.11 30 31 32 33 Even in studies showing the highest rate of complications at 53%, patients were found to have a satisfaction rate of 86%.30
The most common late complication is inadequate resection of glandular tissue or skin. We have found that for patients with type I or type II gynecomastia, underresection of the glandular tissue is the most common aesthetic complaint, particularly following liposuction alone. In such cases, direct excision using an infra-areolar incision can produce quite satisfactory results. Undercorrection is an important topic to discuss with all patients in the preoperative consultation. Regardless of the severity and technique used, a discussion regarding the possible need for a second stage procedure for further resection or revision if or paramount importance.
An important note in second-stage procedures is the differentiation of residual tissue versus recurrence. In the instance of recurrence, a higher suspicion for underlying systemic disease process should be considered. We have experienced a case of recurrence after treatment of liposuction, which was retreated with direct excision revealing malignant pathology of ductal carcinoma in situ.
Conclusion
An increasing number of techniques are available in the modern plastic surgeon's armamentarium for treating gynecomastia. Many patients will obtain adequate correction with less invasive techniques such as ultrasound-assisted liposuction. In patients with significant skin excess or poor skin elasticity, excellent results can be achieved with a single-stage procedure using a combination of UAL, direct excision, and periareolar skin excision to flatten the breast and remove the excess skin and volume. Only patients with the most severe excess skin redundancy require techniques involving an elliptical skin excision or breast amputation with free nipple grafting.
References
- 1.Rohrich R J Ha R Y Kenkel J M Adams W P Jr Classification and management of gynecomastia: defining the role of ultrasound-assisted liposuction Plast Reconstr Surg 20031112909–923., discussion 924–925 [DOI] [PubMed] [Google Scholar]
- 2.Simon B E, Hoffman S, Kahn S. Classification and surgical correction of gynecomastia. Plast Reconstr Surg. 1973;51(1):48–52. doi: 10.1097/00006534-197301000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Letterman G, Schurter M. The surgical correction of gynecomastia. Am Surg. 1969;35(5):322–325. [PubMed] [Google Scholar]
- 4.Monarca C, Rizzo M I. Gynecomastia: tips and tricks-classification and surgical approach. Plast Reconstr Surg. 2013;131(5):863e–865e. doi: 10.1097/PRS.0b013e318287a18f. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg G J. Gynecomastia: suction lipectomy as a contemporary solution. Plast Reconstr Surg. 1987;80(3):379–386. [PubMed] [Google Scholar]
- 6.Böni R. Tumescent power liposuction in the treatment of the enlarged male breast. Dermatology. 2006;213(2):140–143. doi: 10.1159/000093853. [DOI] [PubMed] [Google Scholar]
- 7.Lista F, Ahmad J. Power-assisted liposuction and the pull-through technique for the treatment of gynecomastia. Plast Reconstr Surg. 2008;121(3):740–747. doi: 10.1097/01.prs.0000299907.04502.2f. [DOI] [PubMed] [Google Scholar]
- 8.Gingrass M K, Shermak M A. The treatment of gynecomastia with ultrasound-assisted lipoplasty. Perspect Plast Surg. 1999;12:101. [Google Scholar]
- 9.Hodgson E LB Fruhstorfer B H Malata C M Ultrasonic liposuction in the treatment of gynecomastia Plast Reconstr Surg 20051162646–653., discussion 654–655 [DOI] [PubMed] [Google Scholar]
- 10.de Souza Pinto E B Abdala P C Maciel C M dos Santos FdeP de Souza R P Liposuction and VASER Clin Plast Surg 2006331107–115., vii [DOI] [PubMed] [Google Scholar]
- 11.Wong K Y, Malata C M. Conventional versus ultrasound-assisted liposuction in gynaecomastia surgery: a 13-year review. J Plast Reconstr Aesthet Surg. 2014;67(7):921–926. doi: 10.1016/j.bjps.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Fruhstorfer B H, Malata C M. A systematic approach to the surgical treatment of gynaecomastia. Br J Plast Surg. 2003;56(3):237–246. doi: 10.1016/s0007-1226(03)00111-5. [DOI] [PubMed] [Google Scholar]
- 13.Webster J P. Mastectomy for gynecomastia through a semi-circular incision. Ann Surg. 1946;124:557. [PubMed] [Google Scholar]
- 14.Morselli P G. “Pull-through”: a new technique for breast reduction in gynecomastia. Plast Reconstr Surg. 1996;97(2):450–454. doi: 10.1097/00006534-199602000-00028. [DOI] [PubMed] [Google Scholar]
- 15.Morselli P G, Morellini A. Breast reshaping in gynecomastia by the “pull-through technique”: considerations after 15 years. Eur J Plast Surg. 2012;35(5):365–371. doi: 10.1007/s00238-011-0595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammond D C Arnold J F Simon A M Capraro P A Combined use of ultrasonic liposuction with the pull-through technique for the treatment of gynecomastia Plast Reconstr Surg 20031123891–895., discussion 896–897 [DOI] [PubMed] [Google Scholar]
- 17.Bracaglia R, Fortunato R, Gentileschi S, Seccia A, Farallo E. Our experience with the so-called pull-through technique combined with liposuction for management of gynecomastia. Ann Plast Surg. 2004;53(1):22–26. doi: 10.1097/01.sap.0000106429.37110.cf. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg G J. A new cannula for suction removal of parenchymal tissue of gynecomastia. Plast Reconstr Surg. 1994;94(3):548–551. doi: 10.1097/00006534-199409000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Samdal F, Kleppe G, Aabyholm F. A new suction-assisted device for removing glandular gynecomastia. Plast Reconstr Surg. 1991;87(2):383–385. [PubMed] [Google Scholar]
- 20.Prado A C, Castillo P F. Minimal surgical access to treat gynecomastia with the use of a power-assisted arthroscopic-endoscopic cartilage shaver. Plast Reconstr Surg. 2005;115(3):939–942. doi: 10.1097/01.prs.0000153237.35202.7d. [DOI] [PubMed] [Google Scholar]
- 21.Ohyama T, Takada A, Fujikawa M, Hosokawa K. Endoscope-assisted transaxillary removal of glandular tissue in gynecomastia. Ann Plast Surg. 1998;40(1):62–64. doi: 10.1097/00000637-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Jarrar G, Peel A, Fahmy R, Deol H, Salih V, Mostafa A. Single incision endoscopic surgery for gynaecomastia. J Plast Reconstr Aesthet Surg. 2011;64(9):e231–e236. doi: 10.1016/j.bjps.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Letterman G, Schurter M. Surgical correction of massive gynecomastia. Plast Reconstr Surg. 1972;49(3):259–262. doi: 10.1097/00006534-197203000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Huang T T, Hidalgo J E, Lewis S R. A circumareolar approach in surgical management of gynecomastia. Plast Reconstr Surg. 1982;69(1):35–40. [PubMed] [Google Scholar]
- 25.Botta S A. Alternatives for the surgical correction of severe gynecomastia. Aesthetic Plast Surg. 1998;22(1):65–70. doi: 10.1007/s002669900168. [DOI] [PubMed] [Google Scholar]
- 26.Haddad Filho D, Arruda R G, Alonso N. Treatment of severe gynecomastia (Grade III) by resection of periareolar skin. Aesthet Surg J. 2006;26(6):669–673. doi: 10.1016/j.asj.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Ward C M, Khalid K. Surgical treatment of grade III gynaecomastia. Ann R Coll Surg Engl. 1989;71(4):226–228. [PMC free article] [PubMed] [Google Scholar]
- 28.Gheita A. Gynecomastia: the horizontal ellipse method for its correction. Aesthetic Plast Surg. 2008;32(5):795–801. doi: 10.1007/s00266-008-9190-2. [DOI] [PubMed] [Google Scholar]
- 29.Molina A R, Kokkinos C, Soldin M. Male pseudogynaecomastia following massive weight loss: introducing the superolateral pedicle. J Plast Reconstr Aesthet Surg. 2014;67(1):e25–e26. doi: 10.1016/j.bjps.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Colombo-Benkmann M, Buse B, Stern J, Herfarth C. Indications for and results of surgical therapy for male gynecomastia. Am J Surg. 1999;178(1):60–63. doi: 10.1016/s0002-9610(99)00108-7. [DOI] [PubMed] [Google Scholar]
- 31.Petty P M, Solomon M, Buchel E W, Tran N V. Gynecomastia: evolving paradigm of management and comparison of techniques. Plast Reconstr Surg. 2010;125(5):1301–1308. doi: 10.1097/PRS.0b013e3181d62962. [DOI] [PubMed] [Google Scholar]
- 32.Li C C, Fu J P, Chang S C, Chen T M, Chen S G. Surgical treatment of gynecomastia: complications and outcomes. Ann Plast Surg. 2012;69(5):510–515. doi: 10.1097/SAP.0b013e318222834d. [DOI] [PubMed] [Google Scholar]
- 33.Courtiss E H. Gynecomastia: analysis of 159 patients and current recommendations for treatment. Plast Reconstr Surg. 1987;79(5):740–753. doi: 10.1097/00006534-198705000-00010. [DOI] [PubMed] [Google Scholar]


