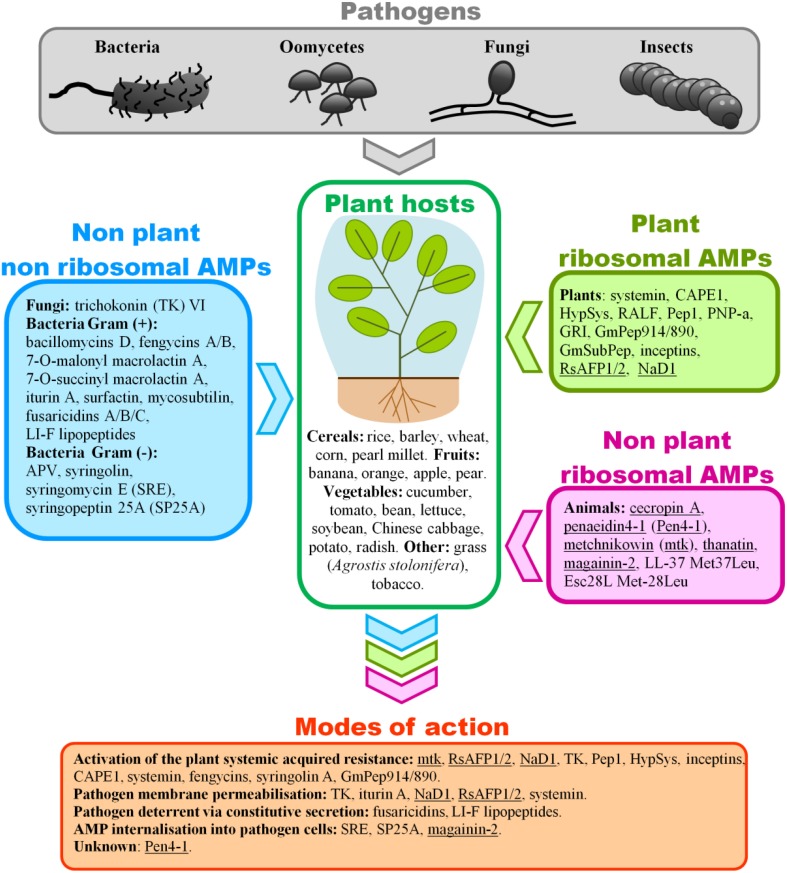
Figure 1.
Summary of the modes of action of non-plant and plant related AMPs involved in host resistance against plant pathogens reviewed here. A number of pests (pathogens and herbivores) can attack a crop which deploys an arsenal of defense to stay healthy. Yet some pests manage to break through plant defense mechanisms. Ribosomal and non-ribosomal AMPs synthesized by organisms other than the crop of interest offer a way to increase the plant resistance levels against pests. Their mode-of-actions, where known, mainly boosts systemic acquired resistance (SAR) of the plant host, particularly through the induction of pathogenesis-related (PR) proteins as reported for mtk, TK, CAPE1, Pep1, as well as the induction of reactive oxygen species (ROS) as reported for RsAFPs, NaD1, TK, Pep1, HypSys. Another manifestation of SAR implicates phytohormones signaling pathways as well as secondary metabolites. A number of AMPs have been associated with salicilic acid (SA) as reported for TK, inceptins, and CAPE1, jasmonic acid (JA) as reported for inceptins, CAPE1, Pep1, and systemin, and ethylene as reported for Pep1. Fengycins have been implicated in the production of phenolic compounds derived from the plant defense-related phenylpropanoid metabolism, while GmPep914/890 enhanced phytoalexin production. Syringolin A was shown to induce the transcription of Pir7b, Pir2, Pir2, and Rir1 defense genes. Another common mode of action in plant defense mechanism operates by compromising the cell membrane permeability of the pathogen. Indeed TK was involved in the change of fungal membrane permeability and disintegration of subcellular structures, while iturin A was associated with the creation of transmembrane channels thus resulting in the release of vital ions such as K+. NaD1, interacting with PIP2, triggered the granulation of the hyphal cytoplasm followed by cell death. The interaction of RsAFP1/2 with fungal membrane glucosylceramide (GlcCer) resulted in the activation of MAP kinase and cell wall integrity signaling pathways. Systemin caused a rapid alkalinization of the extracellular space via blockage of a proton pump in the cell membrane. AMP internalization into pathogen cells can be mediated by cell wall degrading enzymes (CWDEs) as reported for syringomycin E (SRE), syringopeptin 25A (SP25A), and magainin-2. Finally some AMPs act as pathogen deterrent via constitutive secretion as demonstrated for fusaricidins, and LI-F lipopeptides. (1) AMPs used for transgenic experiments in plants are underlined. (2) Pathogens listed are bacteria (Erwinia amylovora, Pseudomonas syringae pathovars, P. aeruginosa, Serratia marcescens, Bacillus subtilis, Pectobacterium carotovorum, Ralstonia solanacearum, Dickeya dadantii), oomycetes (Pythium irregular, P. dissotocum, P. ultimum, P. aphanidermatum, Phytophthora infestans, P. parasitica, P. nicotianae), fungi (Cochliobolis heterostrophus, Colletotrichum graminicola, C. gloeosporioides, C. higginsianum, Rhizoctonia cerealis, R. solani, Fusarium oxysporum pathovars, F. graminearum, F. culmorum, F. verticillioides, Verticillium dahliae, Blumeria graminis, Botrytis cinerea, Penicillium expansum, P. crustosum, Rhodotorula pilimanae, Rhizopus sp., Alternaria citri, Botryosphaeria sp., Fusicoccum aromaticum, Lasiodiplodia theobromae, Sphaerotheca fuliginea, Bremia lactucae, Cladosporium cucumerinum, Ascochyta citrullina, Sclerotinia homoecarpa, Magnaporthe oryzae, Aspergillus flavus, Sclerospora graminicola), and insects (Spodoptera litura, S. frugiperda, grasshopper).