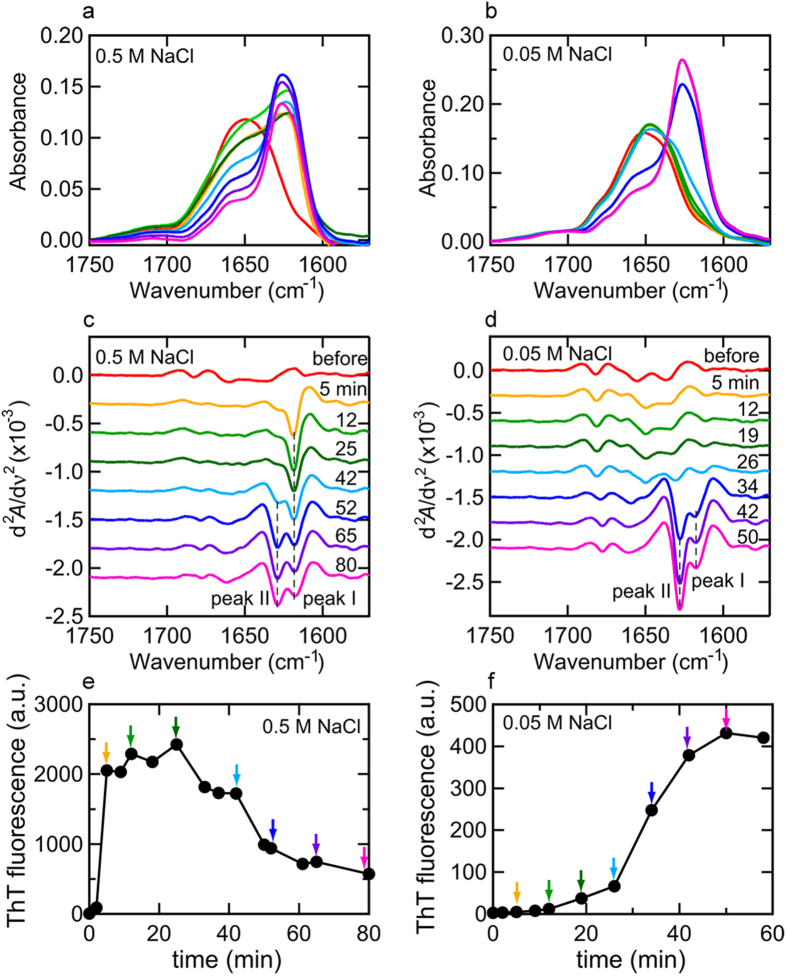
Figure 2.
Time-dependent changes in FTIR absorption spectra at the amide I′ region during the fibrillation reaction in the presence of 0.5 M (a,c,e) and 0.05 M NaCl (b,d,f). (a,b) FTIR absorption spectra at 0.5 M (a) and 0.05 M NaCl (b). At different time points, an aliquot of the reaction solutions was placed into an optical cell and used for the FTIR measurement, which was performed at 25 °C. These measurements were performed in deuterated solution to prevent water interfering with observations of the amide I band region. In panel (a), the spectra monitored at 5 min (orange), 12 min (light green), 25 min (dark green), 42 min (cyan), 52 min (blue), 65 min (purple), and 80 min (magenta) after initiation of the reaction are shown, and in panel (b), the spectra monitored at 5 min (orange), 12 min (light green), 19 min (dark green), 26 min (cyan), 34 min (blue), 42 min (purple), and 50 min (magenta) are shown. The spectrum of insulin before heating was also represented by red lines as a reference for both conditions. Spectra were normalized so that the integrated intensity of the amide I′ band ranging from 1580 to 1750 cm−1 was set to be equal. (c,d) Second-derivative infrared spectra at 0.5 M (c) and 0.05 M NaCl (d). The line colors are the same as those in panels (a,b). The positions of peak I (1618 cm−1) and peak II (1628–1629 cm−1) are indicated by dashed lines. (e,f) Time course of fibrillation of FTIR samples at 0.5 M (e) and 0.05 M NaCl (f) as monitored using ThT fluorescence intensity concurrently with the FTIR analysis. The time points of sampling for the FTIR measurements are labeled by arrows with the same colors as those used in panels (a,b).