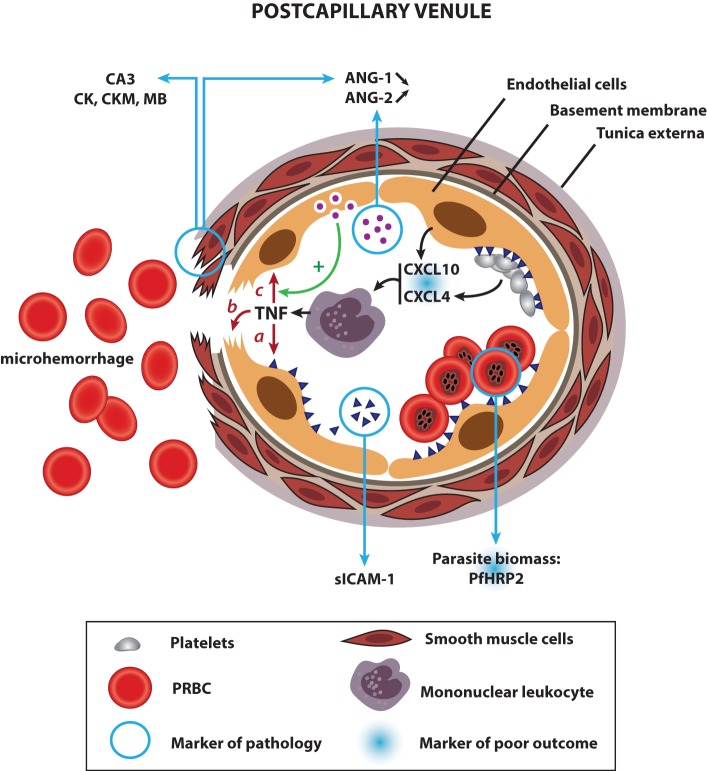
Figure 1.
Links between human CM physiopathology and current biomarkers of pathogenesis and poor outcome: a proposed model. Upon infection with P. falciparum, the host immune system produces pro-inflammatory cytokines, which activate endothelial cells, prompting them to produce CXCL10, a chemoattractant for mononuclear leukocytes. Platelets accumulated in the microvasculature of CM patients release CXCL4 from their alpha granules, which stimulates the production of TNF by mononuclear leukocytes locally. Both cytokines contribute to a hyperinflammatory state in CM and are associated with a poor outcome. Once released, TNF leads to the upregulation of ICAM-1 on endothelial cells (a), which, in turn, induces the sequestration of PRBC and platelets in the cerebral microvasculature. The presence of soluble ICAM-1 in the plasma is reflective of the increase in ICAM-1 levels at the endothelial surface. High densities of sequestered parasites produce elevated amounts of PfHRP2, a marker of pathology and an indicator of poor outcome when detected in the CSF. Sequestration of parasites, coupled with high levels of TNF induce focal vascular injuries, leading to smooth muscle cell damage and ring hemorrhages (b). Injured smooth muscle cells discharge abnormally high amounts of carbonic anhydrase III (CA3), creatine kinase (CK), creatine kinase, muscle (CKM), and myoglobin (MB) in the bloodstream, all biomarkers of CM. In addition, destroyed smooth muscle cells stop producing ANG-1, contributing to its systemic decrease. Coupled with an elevated release of ANG-2 from the Weibel–Palade bodies of activated endothelial cells, the shift in the angiogenic factor balance results in a high ANG-2:ANG-1 ratio, another marker of pathology. ANG-2 sensitizes endothelial cells, which become responsive to sub-threshold concentrations of TNF, contributing to the aggravation of the different pathways described above (c).