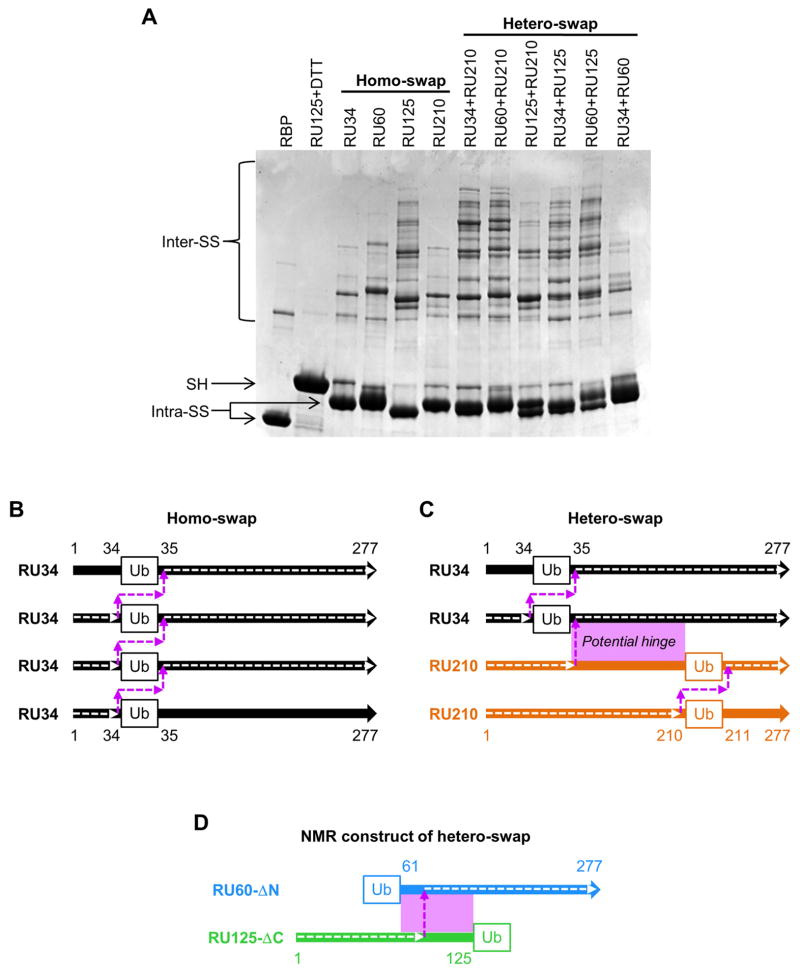
Figure 2. Pairwise mixing of RU-Cys2 variants reveals homo- and hetero-swapping.
(A) Non-reducing SDS-PAGE indicates that all RU-Cys2 variants swap with themselves (lanes labeled homo-swap), but some pairs swap more extensively with each other than with themselves (lanes labeled hetero-swap). The RU34+RU210 and RU60+RU210 lanes contain larger swapped polymers than the RU34, RU60, or RU210 alone lanes. By contrast, the RU34+RU60 lane shows a banding pattern similar to that of the sum of the RU34 and RU60 alone lanes, suggesting that RU34 and RU60 prefer to swap with themselves rather than with each other. (B) and (C) diagram the amino acid connectivities and hinge regions of the proposed homo- and hetero-swapped structures in panel A. The amino acid sequence of the RBP domain is shown as a thick colored arrow and the amino acid connectivity of the swapped complex is traced by a dashed white arrow. The hinge region is a dashed magenta arrow. In the hetero-swapped complex formed by RU34 and RU210 (panel C), the potential hinge region (magenta box) extends from positions 34 to 210. Once the RU34/RU210 hetero-swapped dimer forms, additional RU34 and RU210 proteins can add to the N-terminal and C-terminal ends (respectively; panel C) via the homo-swapping interactions depicted in panel B. (D) Addition of subunits to the heterodimer can be prevented by truncating the amino acids to either side of the Ub domain in the RU hetero-pair.