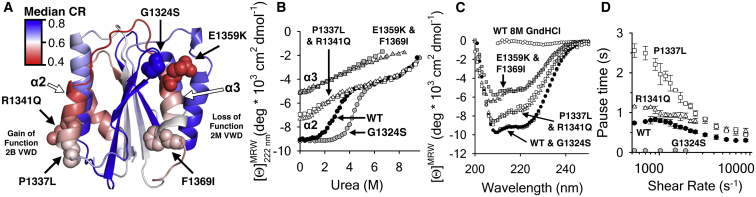
Figure 5.
(A) Head-on view of the GPIbα binding interface of the A1 domain illustrating the location of type-2B VWD mutations (R1341Q and P1337L) in α2 and type-2M mutations in the β-hairpin (G1324S) and α3 (E1359K and F1369I). (B) Urea unfolding of WT (solid circles), G1324S (shaded circles), R1341Q and P1337L (open triangles and squares), and E1359K and F1369I (shaded triangles and squares). (C) Far-UV CD spectra of the protein variants (indictaed in B) in the absence of urea. WT A1 in 8M GndHCl is included as a completely unfolded reference. (D) Shear-dependent platelet pause times on WT (solid circles), R1341Q (triangles), P1337L (squares), and G1324S (shaded circles). The type-2M loss-of-function variants (F1369I and E1359K), did not adhere platelets. Data in (B)–(D) are published in Tischer et al. (4). To see this figure in color, go online.