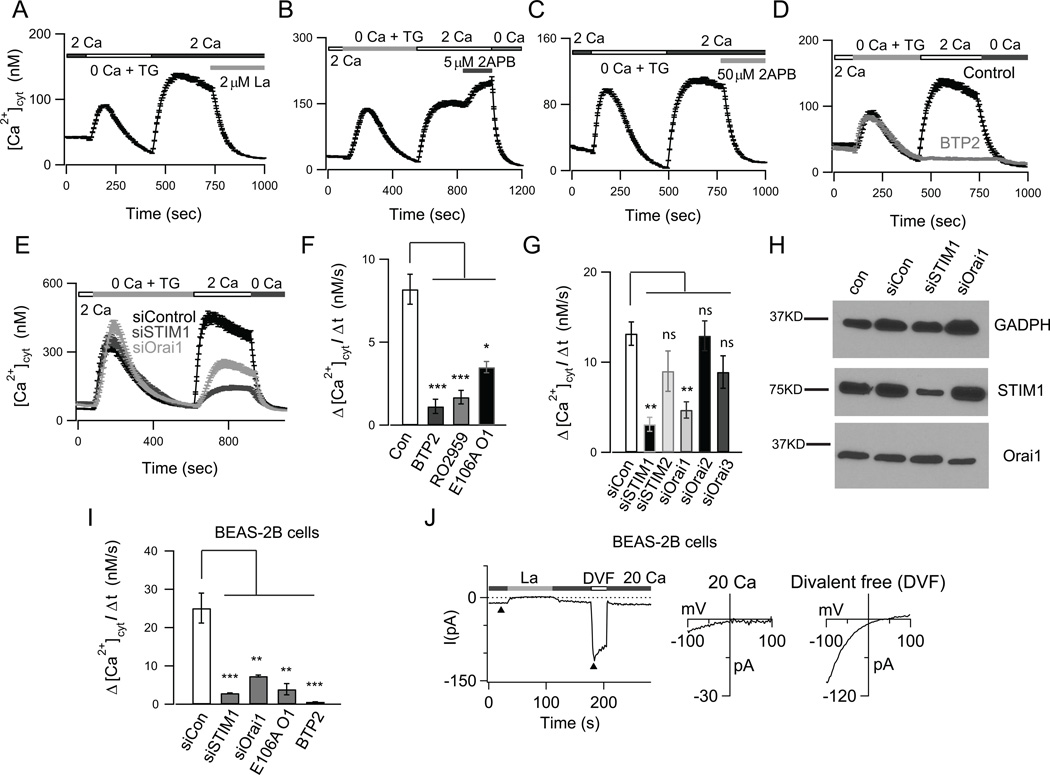
FIGURE 3.
Characterization of store-operated CRAC channels in human airway epithelial cells. (A–C) ER Ca2+ stores were depleted with 1 µM TG in a nominally Ca2+ free solution. Re-addition of 2 mM Ca2+ to the external bath solution evoked SOCE. (A) SOCE is acutely blocked by 2 µM La3+. (B) A low dose of (5 µM) 2-APB facilitated SOCE. (C) A high dose of 2-APB (50 µM) inhibited SOCE. (D) Pre-treatment with the CRAC channel inhibitor BTP2 (500 nM) abolished SOCE in NHBE cells (A-D; mean ± SEM of N=18–29; representative of 3 independent experiments). (E) Knockdown of STIM1 and Orai1 expression in NHBE cells significantly inhibited SOCE (N=21–26 cells; 3 independent experiments). (F–G) Summary of the rates of SOCE in NHBE cells following treatment with the CRAC channel inhibitors BTP2 (500 nM), RO2959 (500 nM) and expression of dominant negative E106A Orai1 (N=21–42 cells; 3 experiments) (F), and following siRNA mediated knockdown of STIM1–2, Orai 1–3 (mean ± SEM of N=22- 34; 3 experiments) (G). (H) Western blots showing expression of Orai1 and STIM1 in NHBE cells. (I) Summary of rates of SOCE in BEAS-2B cells (N=31–48 cells; 3 independent experiments). (J) Whole-cell patch-clamp recordings of ICRAC in BEAS-2B cells. Cells were pretreated with TG to deplete ER Ca2+ stores. The external solution was periodically switched between 20 mM Ca2+ and a divalent-free (DVF solution). In 20 mM Ca2+o, the current exhibits a current-voltage (I-V) relationship with strong inward rectification and positive reversal potential (>+60 mV), consistent with the known biophysical hallmarks of CRAC channels.