Figure 9.
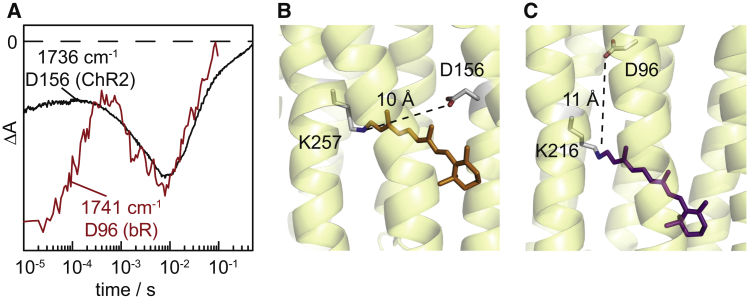
(A) Transient absorption changes of the C=O stretching vibrations of the primary proton donors of D156 of ChR2 and D96 of bR. The kinetics recorded at 1736 cm−1 correspond to D156 of ChR2 (black trace) and is redrawn from Fig. 6. For comparison, the kinetics at 1741 cm−1 of D96 of bR (red) is replotted from Zscherp et al. (53). (B) Structure of channelrhodopsin (PDB:3UG9) including the distance of the internal proton donor D156 to the retinal Schiff base. (C) Structure of bacteriorhodopsin (PDB:1C3W) including the distance of the internal proton donor D96 to the retinal Schiff base. The distances given are from the N of the retinal Schiff base to the carboxylic OD2 of D96 in bR and to the carboxylic OD1 of D195 in the C1C2 chimera (corresponding to D156) of ChR2, respectively. To see this figure in color, go online.