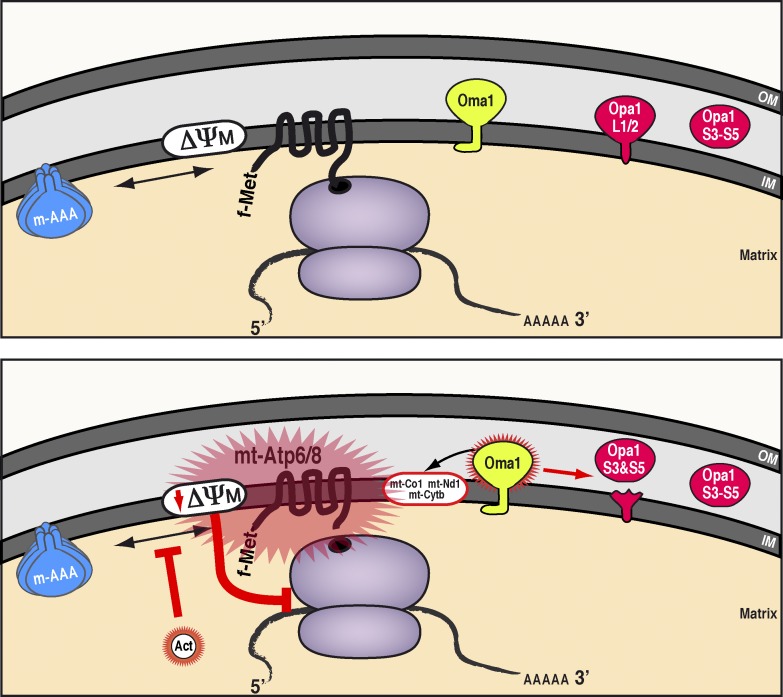
Figure 10.
Model for quality control of mitochondrial protein synthesis and the mechanism for actinonin inhibition. (top) Wild-type scenario in mitochondria: the m-AAA protease ensures de novo proteins synthesized on mito-ribosomes do not overaccumulate in the inner membrane. (bottom) Impairing the turnover of de novo mitochondrial proteins either by actinonin treatment or the loss of m-AAA leads to polypeptide overaccumulation in the inner membrane, in particular the two subunits of the ATP synthase, mt-Atp6 and mt-Atp8. The membrane stress can dissipate the mitochondrial membrane potential, which in turn activates the metalloprotease Oma1 that processes the long Opa1 isoforms required for inner membrane fusion and modulates the abundance of selected de novo mitochondrial proteins. Prolonged disruptions to the mitochondrial inner membrane feed back onto mitochondrial translation elongation by stalling mito-ribosomes and preventing further protein synthesis. IM, inner membrane; ΔΨM, mitochondrial membrane potential; OM, outer membrane.