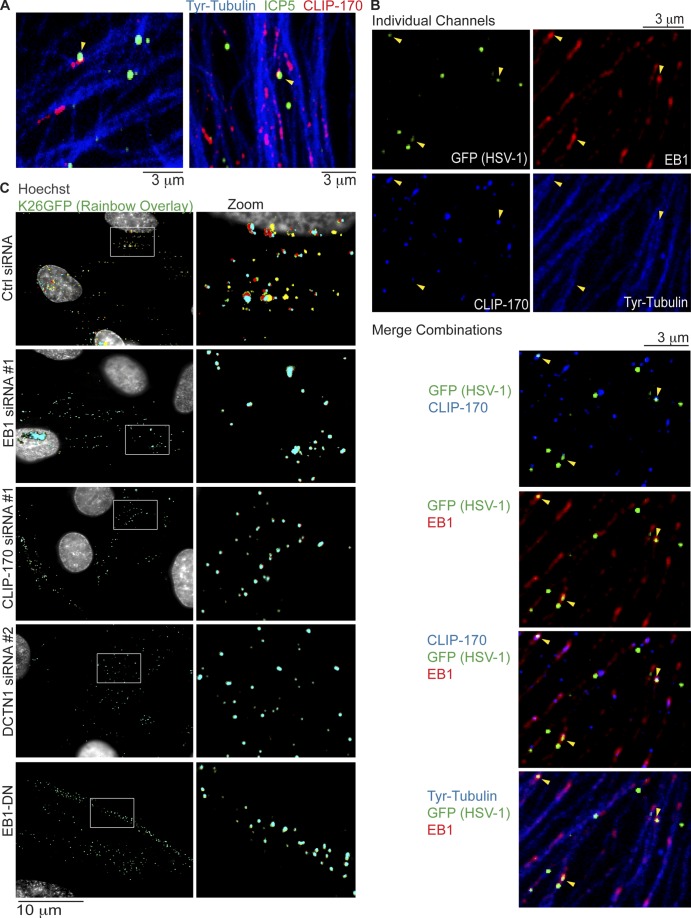
Figure 6.
Retrograde transport of HSV-1 requires EB1, CLIP-170, and DCTN1. (A and B) NHDFs were infected with wild-type HSV-1 or HSV-1 K26GFP at MOI 60 for 30 min. (A) Wild type–-infected samples were stained for the major capsid protein ICP5 (green), CLIP-170 (red), or Tyr-tubulin (blue). Representative confocal images are shown. Arrowheads point to examples of virus particles colocalizing with CLIP-170. (B) K26GFP-infected samples were stained for GFP (green), EB1 (far red), CLIP-170 (red; false-colored blue for merges), or Tyr-tubulin (blue). Representative confocal images are shown, and merge combinations are provided beneath. Arrowheads point to examples of virus particles colocalizing with EB1 and/or CLIP-170. (C) NHDFs were treated with control, EB1, CLIP-170, or DCTN1 siRNAs, or NHDFs expressing EB1-DN were infected with K26GFP at MOI 100 for 3 h. Nuclei were stained using Hoechst, and live cell microscopy was performed capturing images at 1 fps. Rainbow overlays, illustrating particle motility under each condition, were generated using representative frames from Videos 8, 9, and 10, as described in Materials and methods section Antibodies, WB, IF, and imaging. Nuclei were false-colored gray. Magnified images of boxed regions are presented to the right.