Figure 1.
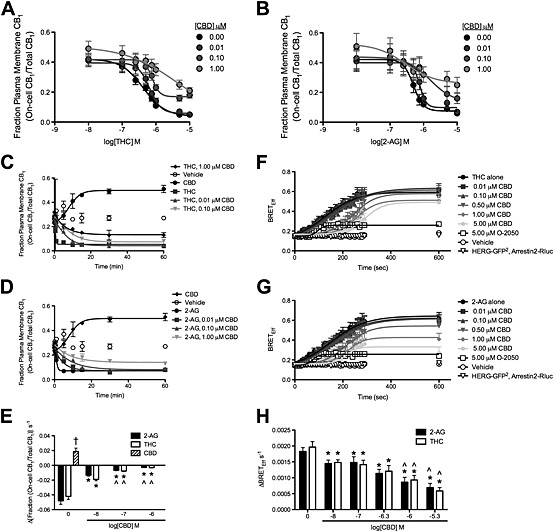
CBD reduced the rate and maximal BRETEff between CB1 receptors and arrestin2 and also the internalization of these receptors in THC‐treated and 2‐AG‐treated STHdh Q7/Q7 cells. (A and B) STHdh Q7/Q7 cells were treated with THC (A) or 2‐AG (B) ± CBD for 10 min, and the fraction of CB1 receptors at the plasma membrane was quantified using On‐cell and In‐cell Western analyses. Data were fit to a nonlinear regression model with variable slope. (C–E) STHdh Q7/Q7 cells were transfected with arrestin2‐Rluc‐containing and CB1‐GFP2‐containing plasmids, and BRET2 was measured every 10 s for 4 min (240 s) and again at 10 min (600 s) after treatment with THC (C) or 2‐AG (D) ± O‐2050 or CBD. Data were fit to a nonlinear regression model with variable slope. (E) The rate of arrestin2 recruitment to CB1 receptors was measured as the change in BRETEff s−1 during the first 4 min. (F–H) STHdh Q7/Q7 cells were treated with THC (F) or 2‐AG (G) ± CBD for 60 min, and the fraction of CB1 receptors at the plasma membrane was quantified using On‐cell and In‐cell Western analyses. Data were fit to a nonlinear regression model with variable slope. (H) The rate of CB1 receptor internalization was measured as the change in the fraction On‐cell CB1/total CB1 min−1 prior to plateau. †P < 0.01 compared with 2‐AG or THC alone, *P < 0.01 compared with 0 CBD within orthosteric ligand treatment, ^P < 0.01 compared with 0.01 μM CBD (log[CBD] M = −8) within orthosteric ligand treatment; two‐way ANOVA with Bonferroni's post hoc test. N = 6.
