Table 1.
Clinical Response Rates for Programmed Death-1 and Programmed Death Ligand-1 Inhibitors Across Solid Tumors
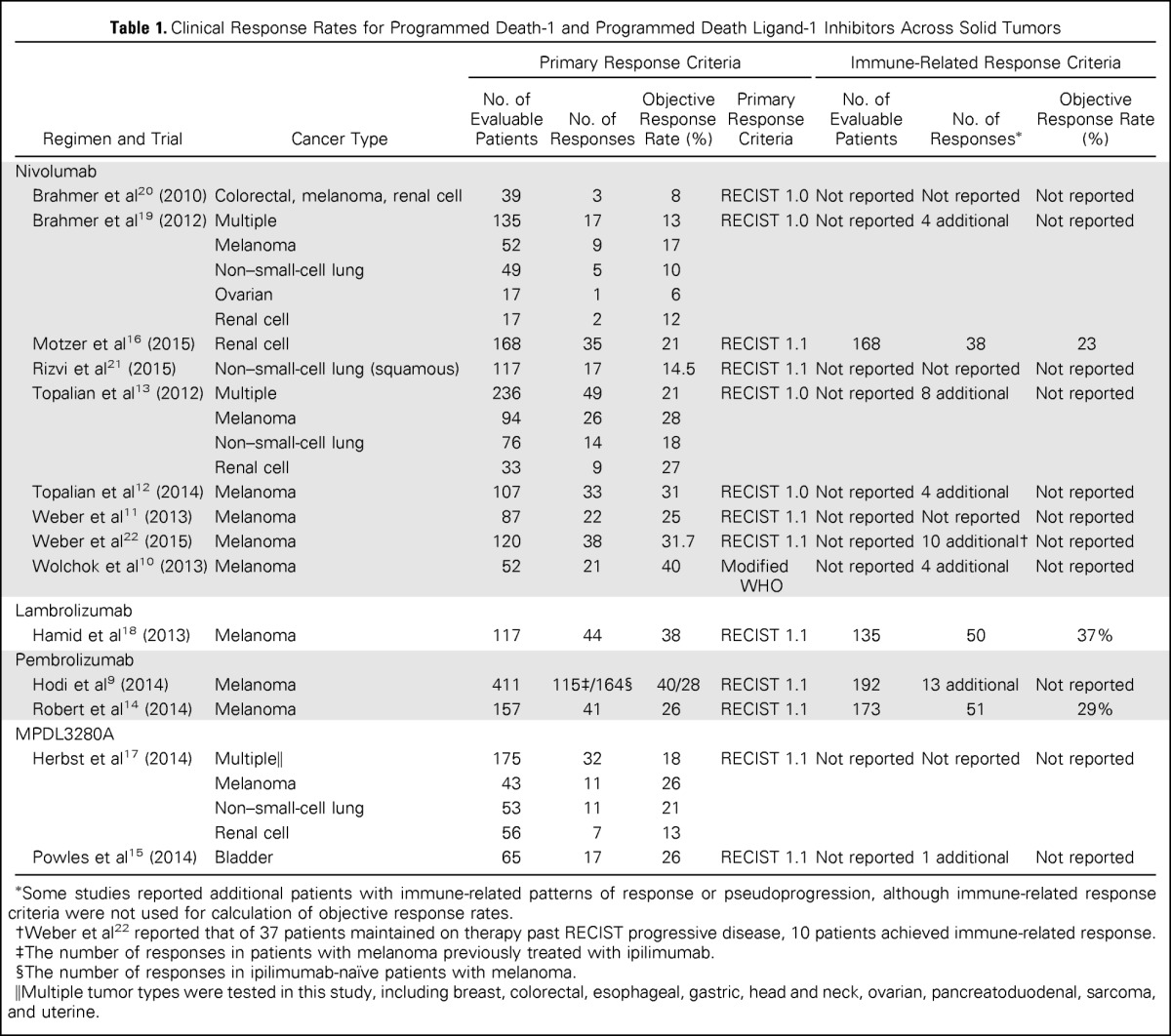
| Regimen and Trial | Cancer Type | Primary Response Criteria |
Immune-Related Response Criteria |
|||||
|---|---|---|---|---|---|---|---|---|
| No. of Evaluable Patients | No. of Responses | Objective Response Rate (%) | Primary Response Criteria | No. of Evaluable Patients | No. of Responses* | Objective Response Rate (%) | ||
| Nivolumab | ||||||||
| Brahmer et al20 (2010) | Colorectal, melanoma, renal cell | 39 | 3 | 8 | RECIST 1.0 | Not reported | Not reported | Not reported |
| Brahmer et al19 (2012) | Multiple | 135 | 17 | 13 | RECIST 1.0 | Not reported | 4 additional | Not reported |
| Melanoma | 52 | 9 | 17 | |||||
| Non–small-cell lung | 49 | 5 | 10 | |||||
| Ovarian | 17 | 1 | 6 | |||||
| Renal cell | 17 | 2 | 12 | |||||
| Motzer et al16 (2015) | Renal cell | 168 | 35 | 21 | RECIST 1.1 | 168 | 38 | 23 |
| Rizvi et al21 (2015) | Non–small-cell lung (squamous) | 117 | 17 | 14.5 | RECIST 1.1 | Not reported | Not reported | Not reported |
| Topalian et al13 (2012) | Multiple | 236 | 49 | 21 | RECIST 1.0 | Not reported | 8 additional | Not reported |
| Melanoma | 94 | 26 | 28 | |||||
| Non–small-cell lung | 76 | 14 | 18 | |||||
| Renal cell | 33 | 9 | 27 | |||||
| Topalian et al12 (2014) | Melanoma | 107 | 33 | 31 | RECIST 1.0 | Not reported | 4 additional | Not reported |
| Weber et al11 (2013) | Melanoma | 87 | 22 | 25 | RECIST 1.1 | Not reported | Not reported | Not reported |
| Weber et al22 (2015) | Melanoma | 120 | 38 | 31.7 | RECIST 1.1 | Not reported | 10 additional† | Not reported |
| Wolchok et al10 (2013) | Melanoma | 52 | 21 | 40 | Modified WHO | Not reported | 4 additional | Not reported |
| Lambrolizumab | ||||||||
| Hamid et al18 (2013) | Melanoma | 117 | 44 | 38 | RECIST 1.1 | 135 | 50 | 37% |
| Pembrolizumab | ||||||||
| Hodi et al9 (2014) | Melanoma | 411 | 115‡/164§ | 40/28 | RECIST 1.1 | 192 | 13 additional | Not reported |
| Robert et al14 (2014) | Melanoma | 157 | 41 | 26 | RECIST 1.1 | 173 | 51 | 29% |
| MPDL3280A | ||||||||
| Herbst et al17 (2014) | Multiple‖ | 175 | 32 | 18 | RECIST 1.1 | Not reported | Not reported | Not reported |
| Melanoma | 43 | 11 | 26 | |||||
| Non–small-cell lung | 53 | 11 | 21 | |||||
| Renal cell | 56 | 7 | 13 | |||||
| Powles et al15 (2014) | Bladder | 65 | 17 | 26 | RECIST 1.1 | Not reported | 1 additional | Not reported |
Some studies reported additional patients with immune-related patterns of response or pseudoprogression, although immune-related response criteria were not used for calculation of objective response rates.
Weber et al22 reported that of 37 patients maintained on therapy past RECIST progressive disease, 10 patients achieved immune-related response.
The number of responses in patients with melanoma previously treated with ipilimumab.
The number of responses in ipilimumab-naïve patients with melanoma.
Multiple tumor types were tested in this study, including breast, colorectal, esophageal, gastric, head and neck, ovarian, pancreatoduodenal, sarcoma, and uterine.
