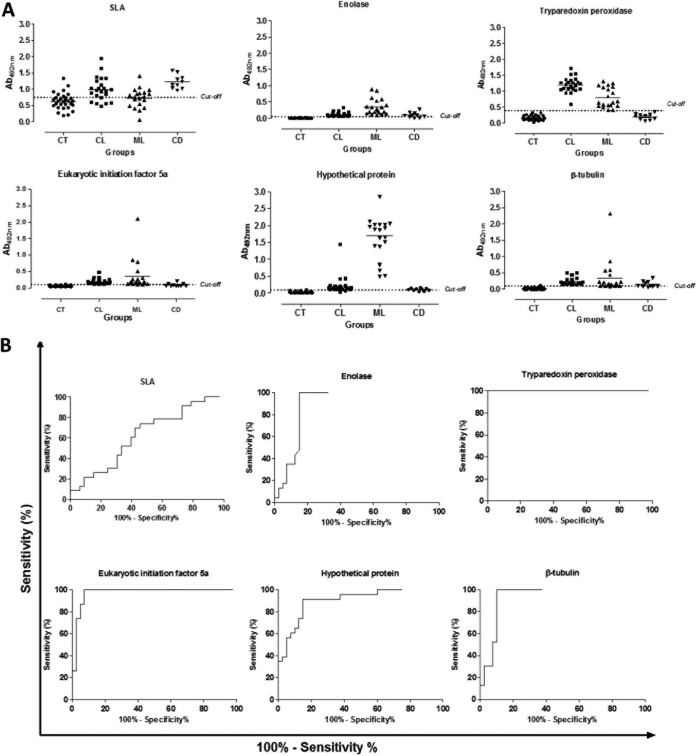
FIG 3.
Diagnostic performance of the recombinant proteins against a human serological panel. Serum samples derived from noninfected subjects living in an area of leishmaniasis endemicity (n = 30), as well as sera from cutaneous (n = 23) or mucosal (n = 20) leishmaniasis patients and sera from Chagas disease patients (n = 10), were used in the ELISAs. Reactivities against enolase (1.0 μg per well), tryparedoxin peroxidase (0.5 μg per well), hypothetical protein (0.5 μg per well), eukaryotic initiation factor 5a (0.05 μg per well), β-tubulin (0.01 μg per well), and L. braziliensis SLA (2.0 μg per well) were determined. The mean optical density (OD) value was calculated by subtracting the mean blank OD from the OD mean for each sample by using the values obtained in the ELISAs (A). The cutoff value for each diagnostic antigen was established based on their sensitivity and specificity values, which were obtained by an ROC analysis (B).