Figure 4.
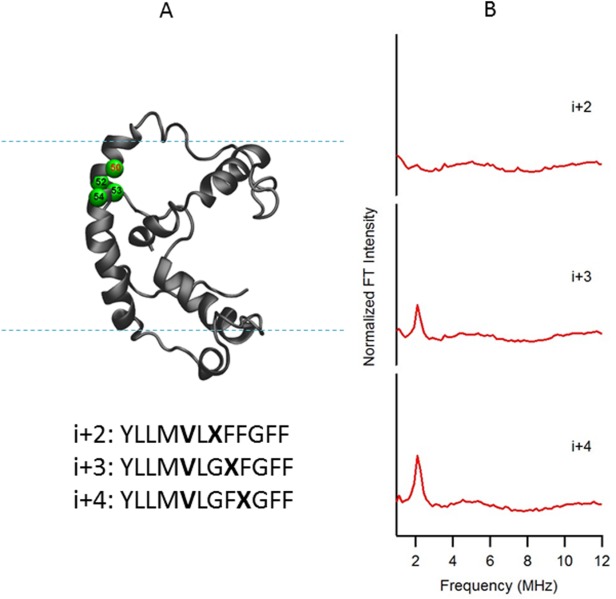
(A) Structural representation of KCNE1 in a DMPC/DPC bicelle (q=3.2). The probed α-helical region is colored in green and located on the transmembrane domain of the full-length KCNE1. Residue 50 is side chain 2H-labeled Val (denoted i), Residues 52, 53, and 54 are independent Cys mutations (denoted i + 2, i + 3, and i + 4, respectively), which is subject to MTSL labeling. Sequences of fully engineered ESEEM mutants around probed regions were shown below the protein model. The targeted 2H-labeled Val is shown in boldface as V, and the MTSL spin-labeled cysteine is shown in boldface as X. (B) Frequency domain spectra of three-pulse ESEEM data of i + 2, i + 3, and i + 4 samples normalized in FT intensity.
