Figure 12.
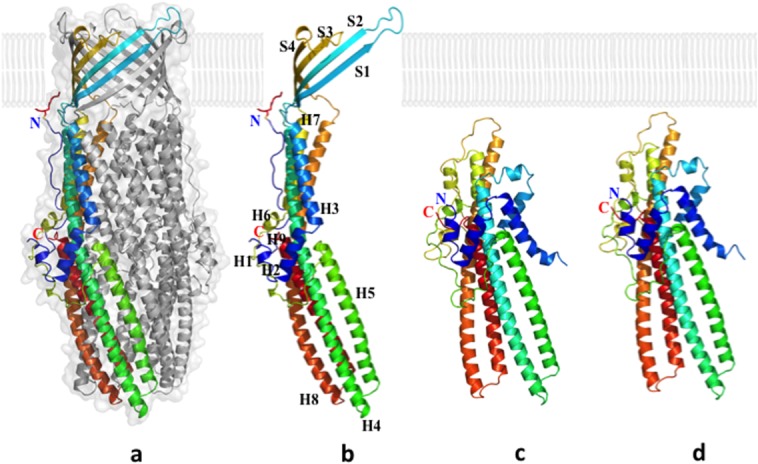
Crystal structures of the CusC outer membrane channel. (a) One monomer of the CusC trimer is shown in rainbow colors, while the other two monomers are depicted in gray. (b) Each CusC monomer (rainbow) can be divided into four β-strands and nine α-helices. The CusC protomer is acylated (red) through the residue C1 to anchor to the outer membrane. (c) Structure of the monomeric ΔC1 mutant. (d) Structure of the monomeric C1S mutant. The mutant structures are seen to adopt a dramatically different, partially folded conformation, compared with the wild-type CusC.
