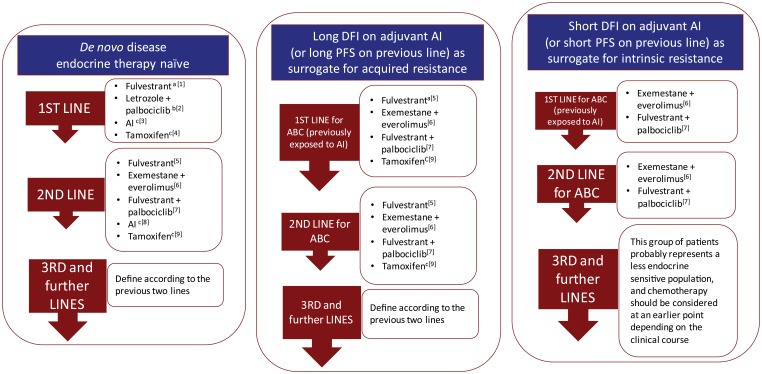
Figure 1.
Suggested endocrine therapy sequencing alternatives in patients with HR+ advanced breast cancer.
General comments to interpret Figure 1
- The sequence of the treatment alternatives in each line text box does not represent a particular preference order.
- Subsequent use of ET should always take into account previous lines of treatment as well as the type and duration of response to previous ET.
- Intrinsic or primary resistance has been defined as recurrence within the first 2 years of adjuvant ET or progressive disease within 6 months of starting ET in the advanced setting.
- Acquired or secondary resistance has been defined as recurrence after the first 2 years of adjuvant ET or disease progression more than 6 months after initiation of ET in the advanced setting. These definitions, although imperfect and somewhat arbitrary, have been useful in some clinical trials to analyze and stratify patient populations.
- These suggestions refer to postmenopausal patients. Specific management of the premenopausal population is addressed in the text.
- These suggestions do not take into consideration acess and regulatory issues in the dfferent regions of the world.
Specific comments
aFulvestrant (500 mg) use in the first-line treatment of HR+ ABC is based on the randomized phase II FIRST trial. The ongoing confirmatory phase III FALCON trial has completed accrual.
bLetrozole in combination with palbociclib in the first-line treatment of HR+ ABC is based on the randomized phase II PALOMA-1 trial. The ongoing confirmatory phase III PALOMA 2 trial has completed accrual.
cDespite the evidence of superior outcomes with other alternatives, our personal opinion is that an AI or tamoxifen remain reasonable options as first-line or second-line ET for selected patients with HR+ ABC, particularly in the very endocrine sensitive population.
dThis combination demonstrated significant PFS advantage in a second-line phase III trial.
eIn this setting patients may have more endocrine resistant disease and would probably benefit more from treatment strategies that could modulate endocrine resistance rather than sequential single agent ET.
fThe sequential use of the combinations with CDK4/6 inhibitors and mTOR inhibitors has not been addressed in clinical trials and there is no definitive information on response rates or benefit in this setting. Chemotherapy remains an alternative for these most endocrine resistant situations. However, the occasional patient that remains clinically stable with slowly progressive disease in spite previous ET failure could still be considered for treatment with a combination with CDK4/6 or mTOR inhibitor depending on previous exposure.
ABC, advanced breast cancer; AI, aromatase inhibitor; CDK, cyclin-dependent kinase; DFI, disease-free interval; ET, endocrine therapy; HR, hormone receptor; mTOR, mammalian target of rapamycin; PFS, progression-free survival;