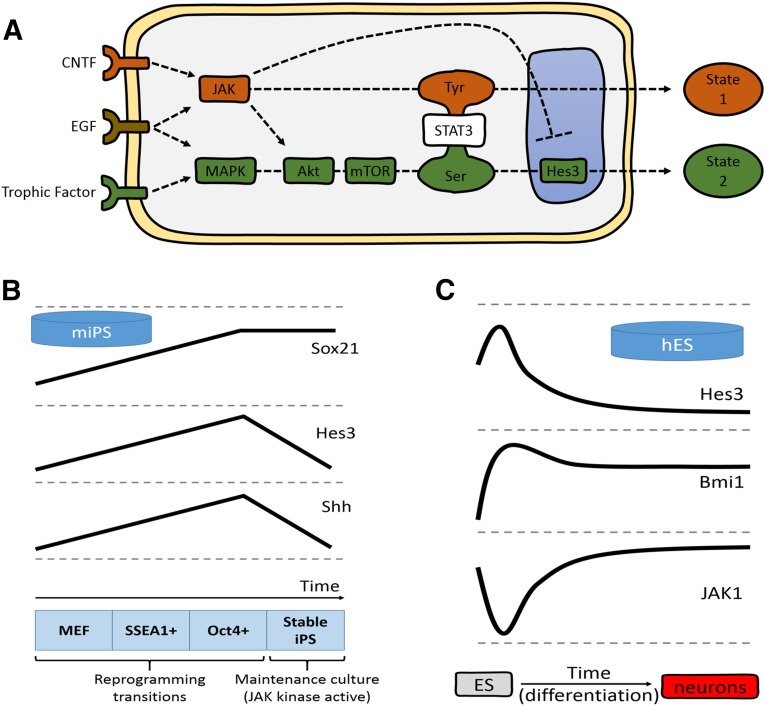
Figure 1.
Noncanonical signaling pathway regulation during reprogramming. (A): Extracellular factors lead to the phosphorylation of STAT3-Tyr via JAK activation or STAT3-Ser via MAPK, Akt, and mTOR activation, and subsequent Hes3 transcription. The two pathways are opposing (e.g., JAK activity in neural stem cells [NSCs] suppresses induction of Hes3). Some cell types (e.g., primary NSCs) are confined to using the STAT3-Ser branch, because the STAT3-Tyr branch leads to their irreversible differentiation. Other cell types (e.g., primary cancer stem cells from glioblastoma multiforme patients and MIN6 cells) grow effectively using either pathway and, through repeated changes in cell culture conditions, can switch their signaling state back and forth. (B): Genes in the STAT3-Ser/Hes3 signaling axis are regulated during mouse fibroblast reprogramming. Sox21, Hes3, and Shh gene expression increases as MEFs transition to SSEA1+ and then to Oct4+ populations during reprogramming to the pluripotent state. Hes3 and Shh are downregulated in resultant stable mouse iPS cells grown in culture conditions that activate JAK (lines not to scale; expression levels at the MEF stage normalized to help visualize patterns and trends). (C): Genes in the STAT3-Ser/Hes3 signaling axis are regulated during neural specification of hES cells. The diagram summarizes the expression patterns of Hes3, Bmi1, and JAK1 over the course of a 77-day protocol to differentiate the human ES cell line WA09 to dorsal telencephalic neuronal fates (lines not to scale; expression levels at day 0 of ES cell stage normalized to help visualize patterns and trends). (B, C): The concepts shown are from gene expression data previously published and reanalyzed for the purposes of the present report [25]. Abbreviations: CNTF, ciliary neurotrophic factor; EGF, epidermal growth factor; hES, human embryonic stem (cell); Hes3, hairy and enhancer of split 3; JAK, Janus kinase; MAPK, mitogen-activated protein kinase; MEF, mouse embryonic fibroblasts; mIPS, mouse induced pluripotent stem (cell); SSEA1, stage-specific embryonic antigen 1; Shh, sonic hedgehog; STAT3, signal transducer and activator of transcription 3.