Abstract
This review focuses on the energy metabolism during pollen maturation and tube growth and updates current knowledge. Pollen tube growth is essential for male reproductive success and extremely fast. Therefore, pollen development and tube growth are high energy-demanding processes. During the last years, various publications (including research papers and reviews) emphasize the importance of mitochondrial respiration and fermentation during male gametogenesis and pollen tube elongation. These pathways obviously contribute to satisfy the high energy demand, and there are many studies which suggest that respiration and fermentation are the only pathways to generate the needed energy. Here, we review data which show for the first time that in addition plastidial glycolysis and the balancing of the ATP/NAD(P)H ratio (by malate valves and NAD+ biosynthesis) contribute to satisfy the energy demand during pollen development. Although the importance of energy generation by plastids was discounted during the last years (possibly due to the controversial opinion about their existence in pollen grains and pollen tubes), the available data underline their prime role during pollen maturation and tube growth.
Keywords: energy metabolism, plastidial glycolysis, pollen tube growth, respiration, malate
Abbreviations
- 2-OG
2-oxoglutarate
- 2-PGA
2-phosphoglycerate
- 3-PGA
3-phosphoglycerate
- ACS
acetyl-CoA synthase
- ADH
alcohol dehydrogenase
- ALDH
aldehyde dehydrogenase
- AOX
alternative oxidase
- BPGA
bisphosphoglyceric acid
- ENO
enolase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- Gln
glutamine
- Glu
glutamate
- GOGAT
glutamate synthase
- GPT
G-6-P/phosphate translocators
- MDH
malate dehydrogenase
- NDP
nucleotide diphosphate kinase
- NMNAT
nicotinate/nicotinamide mononucleotide adenyltransferase
- NTT
ATP/ADP transporters
- OAA
oxaloacetate
- OPP
oxidative pentose-phosphate pathway
- PDC
pyruvate decarboxylase
- PDH
pyruvate dehydrogenase
- PEP
phosphoenolpyruvate
- PGAM
phosphoglycerate mutase
- PGDH
3-phosphoglycerate dehydrogenase
- PK
pyruvate kinase
- PPSB
phosphorylated pathway of serine biosynthesis
- PPT
phosphoenolpyruvate/phosphate translocator
- PSP
phosphoserine phosphatase
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RPOT
T3/T7 phage-type RNA polymerases
- T
malate/oxaloacetate translocator
- TP
triose phosphate
Introduction
Sexual reproduction of flowering plants comprises pollination, pollen tube growth and double fertilization. Pollen grains are produced within the anther and released to the environment after maturation. The primary function of a pollen grain is the delivery of sperm cells to the female gametophyte. The first step of pollination is the adhesion of a pollen grain on the stigma. Pollen grains are transferred to the stigma via insects, birds, animals, wind or direct contact. Pollen hydration, germination and pollen tube growth through the pistil are the next steps. Within the pistil, chemical gradients produced by the female diploid tissue support pollen tube growth and guidance.1-4 In addition, signal molecules released by the female gametophyte can act as short-range attractants which guide the pollen tubes into the embryo sac.5-10 The tip of the pollen tube then enters the ovary and penetrates through the micropyle opening in the ovule. Sperm cells are delivered to the embryo sac of the ovary through the pollen tube.11,12 During double fertilization, one sperm cell fertilizes the egg cell to form the zygote, while the other sperm cell fuses with the 2 polar nuclei of the large central cell that then forms the triploid endosperm.
The total number of pollen grains on a stigma often exceeds the number that is necessary to fertilize all ovules. Therefore, a competitive pollen tube growth takes place in the pistil. In higher plants, pollen tube growth is highly polarized and extremely fast. Indeed, the pollen tube is the fastest growing plant cell known. Rates of pollen tube growth can reach μm s−1 in various plant species (e.g. 2.8 μm s−1 in maize and 0.2-0.3 μm s−1 in lily, calculated from published data).13-15 This rapid elongation of pollen tubes is essential for male reproductive success.16 Consequently, pollen development and tube growth are high ATP-consuming processes.
Up to date, mainly mitochondrial respiration and fermentation were considered to play an important role for energy generation during pollen tube growth, and plastids were discounted. This was possibly due to the controversial opinion about the existence of plastids within pollen grains and pollen tubes. In this review, we focus on current knowledge and speculate about possible future attempts to find out more about the importance of plastids in pollen grains and pollen tube growth with regard to the generation of energy needed for the rapid growth rate during elongation.
The role of mitochondrial respiration and fermentation during pollen development and tube growth
In eukaryotic cells in general, mitochondria are considered to be the major organelles for energy production. During respiration, the electron transfer through Complexes I, III and IV is coupled to proton translocation from the matrix to the intermembrane space which builds up a membrane potential and some pH gradient, the “proton motif force.” The mitochondrial membrane potential represents the force for ATP generation via ATP synthase.
In the literature, the mitochondrial respiratory chain and the resulting ATP are highly discussed as the major source for energy supply during pollen development and tube growth. Indeed, pollen contain approximately 20 times more mitochondria per cell in maize 17 and respire 10 times faster than normal vegetative tissues in lily and tobacco.18-20 Oxygen consumption measurements of lily pollen revealed that respiration for pollen tube growth can be divided into 3 phases.18 In phase 1 (prior to the emergence of the pollen tube tip), respiration rapidly increases for approximately 30 minutes. Afterwards, while the initiation of pollen germination and growth occurs (phase 2), the respiration rate decreases to approximately 40% of the initial rate. The final phase represents the stage of highest respiration. During this phase, pollen tube growth continues, and the respiration rate is twice the rate of phase 2.18
Interestingly, a comparison of trinucleate and binucleate pollen grain respiration revealed that trinucleate pollen, which are considered to be more evolved, 21 consume 2 to 3 times more oxygen than binucleate pollen.22 Although a difference in respiration between trinucleate and binucleate pollen could be observed, there was no correlation between oxygen consumption and pollen germination or tube growth. This is possibly associated with the early division of the generative cell during mitosis II, when 2 sperm cells are formed in tricellular species.23
Besides the high respiration rate during pollen development and tube growth, a high accumulation of mitochondria can be observed near to the subapical area of the pollen tube (clear zone) and on the periphery of the cells (Fig. 1).23-26 This localization is thought to be related to the need of local ATP production for cortically occurring energy-consuming processes like endocytosis and exocytosis, the maintenance of the stability of the actin cytoskeleton and the functioning of ATPases associated to the plasma membrane.23,27-30
Figure 1.
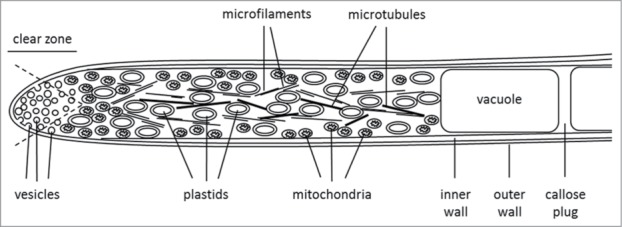
Scheme for the spacial distribution of plastids and mitochondria in pollen tubes.
During the last years, various research groups focused on transcriptome and proteome profiling of different stages of pollen grain maturation and pollen tube growth.31-38 Approximately half of the identified proteins and transcripts are involved in metabolism, energy generation and cell structure emphasizing the important roles for energy metabolism-related proteins in pollen germination and rapid pollen tube growth. The proteomic data suggest that no alternative oxidase (AOX) protein is present in pollen.39,40 Based on these observations it was concluded that a shunt in the electron transport chain during abiotic and biotic stress operated by AOX is not needed during pollen development and tube growth suggesting that instead a full-on energy-generating respiration is essential.41 This hypothesis is inconsistent with the fact that AOX1A and AOX1B transcripts could be detected in Arabidopsis thaliana pollen following germination and tube elongation.36 In addition, there is some evidence for the involvement of AOX in pollen tube development. Various research groups showed that AOX is important for plant reproduction. Especially during microsporogenesis and microgametogenesis, AOX seems to play an essential role.42 Furthermore, AOX was found to be strongly produced within the tapetum and developing microspores of a fertile petunia line but not in a cytoplasmic male-sterile line.43 Also an increased number of pollen abortions could be observed when AOX antisense plants of tobacco and soybean were analyzed.44-46
Contrary to what might be expected, oxidative phosphorylation possibly is not the major pathway for oxygen consumption and energy supply during pollen development and tube growth, because e.g., oligomycin had only little effect on the high respiratory rates of germinating pollen.22 It was also shown that pollen tube growth in total is much less sensitive to respiratory inhibition than is respiration.47 This is possibly due to the dynamic adaption of metabolic pathways (e.g., glycolysis) during pollen germination and tube growth after the inhibition of the mitochondrial electron transport chain.48 Therefore, alternative pathways within developing pollen and elongating pollen tubes seem to be present to meet the pollen tube´s energy demand under all kinds of conditions. In this context, fermentation is often discussed as an alternative pathway for ethanol and ATP production when oxygen is low.41
Under low oxygen conditions, fermentation serves to recycle NADH from glycolysis to regenerate NAD+ when oxidative phosphorylation is impaired. An oxygen gradient occurs within the style of an unpollinated stigma. A high oxygen pressure could be detected in the stigma and style which decreases at the base of the style and approaches approximately zero in the ovary. It was also observed that pollen tubes of several species show a positive tropic response toward O2 in an oxygen gradient in a colloidal medium which mimics the stylar environment 49 and that the pollen tube itself additionally creates hypoxic regions within the style.50
Tobacco and petunia pollen were shown to produce copious amounts of ethanol even in the absence of oxygen stress 51 indicating that fermentation plays an important role during pollen-pistil interaction. In pollen, ethanol is produced simultaneously with an extremely high respiration rate and the flux to ethanol is regulated by sugar availability, not by oxygen as it is the case in vegetative tissues.20,51,52
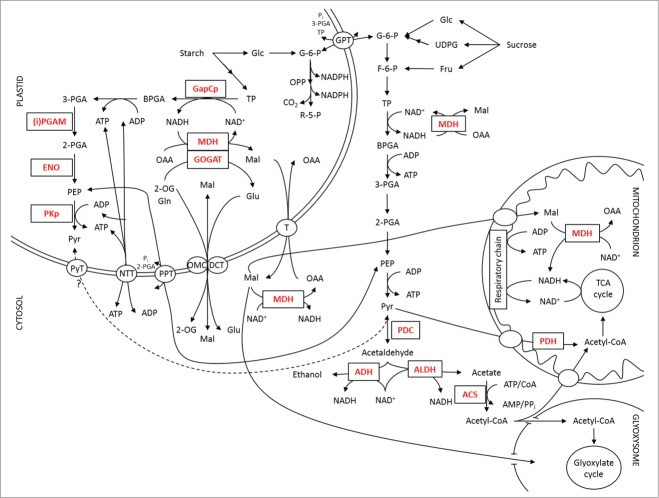
Ethanolic fermentation involves 2 enzymes: The pyruvate decarboxylase (PDC) which is responsible for the conversion of pyruvate into acetaldehyde and CO2, and the alcohol dehydrogenase (ADH) which converts acetaldehyde into ethanol and thereby regenerates NAD+ (Fig. 2). If ethanolic fermentation represents an important metabolic pathway during pollen tube development, a knock-out of this gene should result in impaired pollen development or tube growth. However, heterozygous knock-out mutants for adh segregate in a normal Mendelian ratio, and pollen development and tube growth are not affected by an adh knock-out,53 indicating that ethanolic fermentation possibly is not the main pathway for the regeneration of NAD+.
Figure 2.
Pathways of energy metabolism in pollen tubes.
Various research groups have identified an alternative metabolic route to fermentation for providing TCA cycle substrates, the pyruvate dehydrogenase (PDH) bypass. In this pathway pyruvate is converted to acetaldehyde (catalyzed by PDC) which is then oxidized to acetate (catalyzed by aldehyde dehydrogenase; ALDH). Finally, acetate is converted to acetyl-CoA (catalyzed by acetyl-CoA synthase; ACS) which can enter the TCA cycle and therefore support energy generation via respiration (Fig. 2).54-56 Analysis of a null mutant in pollen-specific pdc2 of Petunia hybrida revealed that the PDC protein and therefore the PDH bypass is the critical pathway for pollen tube growth rather than ADH. Pollen tube growth through the style is decreased when pdc2 is lacking. In addition, the mutant allele shows impaired transmission through the male gametophyte, when there is competition between wild-type and knock-out pollen.56 These results clearly show that the PDH bypass, and not fermentation, is critical for efficient pollen tube growth.
However, the PDH bypass provides only a partial answer concerning the energy generation in growing pollen tubes because the ADH and PDC are present in pollen of bicellular species but have no representation in tricellular pollen (e.g., Arabidopsis).23 For this reason, it is still unclear where energy generation takes place and which protein could be responsible for the regeneration of NAD+.
Plastids and plastidial glycolysis during pollen development
Up to now, there is a controversial opinion about the existence of plastids within pollen grains and pollen tubes. However, various data are available now which focus on the visualization and dynamics of plastids within pollen.57-59 The vegetative cell in pollen, which produces the pollen tube, contains plastids which accumulate starch whereas the generative cell is devoid of plastid DNA.60,61 At the initial stage of pollen formation, plastids in the vegetative cell are poorly differentiated and possess an indistinguishable inner membrane. In contrast, the structure of plastids in mature pollen contains a double membrane structure with several starch grains and simple thylakoid membranes.62 During pollen germination, the majority of plastids are retained in the pollen grain, whereas some plastids migrate throughout the pollen tube. During the progression of pollen tube growth, the number of plastids in the pollen tube increases and the majority of plastids shift to the tube. Within the pollen tube, plastids are distributed in both central and peripheral regions but are not localized to the “clear zone” (Fig. 1).58 The localization of plastids in pollen is consistent with the distribution of mitochondria. Nevertheless, it was shown that GFP signals of plastids do not overlap with RFP signals from mitochondria.57
Although the number of plastids in pollen is relatively low compared to other organelles, 57,59 the energy production via glycolysis within plastids contributes to the generation of energy needed for the rapid growth during pollen tube elongation. During the last years, various research articles were published where it was shown that a knock-out of genes coding for plastid-localized glycolytic enzymes and related proteins specifically affect male gametophyte development.63-66
During the final stage of pollen maturation, starch biosynthesis is critical because starch represents a source of energy for pollen germination and elongation and, in addition, serves as a checkpoint of pollen maturity (e.g., it was shown that pollen non-viability is associated with starch deficiency).67-69 Starch degradation and following triose-phosphate oxidation with coupled ATP generation in plastids require a continuous regeneration of NAD+ (Fig. 2). During glycolysis, NADH is produced by the activity of the bispecific NAD(P)-dependent glyceraldehyde-3-phosphate dehydrogenase (GAPDH; GAP A/B), which is active with NAD+ in the dark, or by the plastid-localized NAD-GAPDH (GapCp).64,70-72 In both cases, the regeneration of NAD+ is catalyzed by the plastid-localized NAD-dependent malate dehydrogenase (plNAD-MDH) or alternatively by NADH-GOGAT.66,73,74 A knock-out of gapcp1 and gapcp2 leads to male sterility in Arabidopsis thaliana indicating the important role of plastidial glycolysis during pollen development. Pollen of gapcp1gapcp2 double mutants display shrunken and collapsed forms and are unable to germinate in vitro. In addition, it was shown that these null mutants possess a disorganized tapetum layer which accompanies the down-regulation of genes involved in tapetum development.64
Interestingly, pollen of heterozygous knock-out mutants of plnad-mdh, which is responsible for the removal of NADH generated by GapCp, exhibit an abolished pollen tube growth phenotype in vitro. However, the heavily disturbed pollen tube growth in plnad-mdh mutants can be compensated by adding the NADH-GOGAT substrates 2-oxoglutarate (2-OG) and glutamine (Gln) to the germination medium.66 Due to the fact that the reciprocal crossing of wild-type plants with pollen of heterozygous plnad-mdh knock-out plants generates 50% heterozygous mutants in the progeny, it is assumed that the maternal tissue in the transducing tract of the style can supply the NADH-GOGAT substrates and therefore allows pollen tube elongation in vivo. Although pollen tube growth is not impaired in vivo, the in vitro assay demonstrates the importance of plNAD-MDH for regenerating NAD+ to ensure energy supply by plastidial glycolysis needed for proper pollen tube growth.66
Another plastidial glycolytic enzyme downstream of GapCp is the phosphoglycerate mutase (PGAM). This enzyme catalyzes the reversible interconversion of 3-phosphoglycerate (3-PGA) to 2-phosphoglycerate (2-PGA) (Fig. 2). PGAMs are divided into 2 groups based on their requirement for 2,3-bisphosphoglycerate as a cofactor: cofactor-dependent PGAMs (dPGAM) and cofactor-independent PGAMs (iPGAM).65 Similar to gapcp1gapcp2 double mutants, also the knock-out of ipgam1 and ipgam2 leads to male sterility. In contrast to gapcp1gapcp2 knock-out plants, ipgam1ipgam2 knock-out mutants fully fail to produce pollen and therefore, no pollen grains can be detected within the anthers.65
Further downstream of PGAM the dehydration of 2-PGA to phosphoenolpyruvate (PEP) occurs (Fig. 2). This reaction is catalyzed by enolase (ENO). ENO1 is involved in glycolytic PEP provision in pollen plastids which acts as a precursor for various metabolic pathways indicating its important role in plant metabolism.63 Surprisingly, the knock-out of eno1 causes distorted trichomes and reduced numbers of root hairs, but has no influence on male gametophyte development.75 This is possibly due to the provision of PEP from the cytosol by the phosphoenolpyruvate/phosphate translocator (PPT) which is localized in the plastid envelope (Fig. 2). However, a heterozygous knock-out for eno1 in the homozygous cue1 (defective in PPT1) background (ccEe) possesses a reduced number of pollen containing a large portion of underdeveloped, non-vital, non-germinating pollen grains.63 In addition, some of the mutant pollen accumulate a massive amount of starch in plastids indicating that starch degradation and subsequent glycolysis is inhibited. Therefore, no ATP can be generated via plastidial glycolysis to satisfy the high energy demand during pollen development and elongation.
Based on plastidial glycolysis, different ways for energy generation can be assumed. For example, ATP can be directly exported from plastids via ATP/ADP transporters (NTT) 76 and finally imported into mitochondria. Furthermore, G-6-P can be transported into the cytosol by G-6-P/phosphate translocators (GPT) and enter cytosolic glycolysis to generate ATP and pyruvate. But pyruvate can also directly be exported from plastids (Fig. 2). In the cytosol, the decarboxylation of pyruvate to acetate takes place. Finally, acetate is converted to acetyl-CoA which can in turn enter mitochondria. Alternatively, pyruvate directly enters the mitochondrion where it is converted to acetyl-CoA by PDH which can enter the TCA cycle and further support energy generation by respiration (Fig. 2). Naturally, more profound and precise studies are still missing, but plastidial glycolysis should definitively be included in the discussions about energy metabolism during pollen development and tube growth.
Redox homeostasis during pollen tube elongation
NAD(P)H and ATP are required for all major energy-consuming reactions within a plant cell. Since plant membranes are broadly impermeable to NAD(P) and NAD(P)H, they possess specific translocators (T; Fig. 2) which are responsible for the exchange of malate and oxaloacetate (OAA) enabling the indirect transport of reducing equivalents between different cell compartments. Accordingly, malate valves act as powerful systems for balancing the required ATP/NAD(P)H ratio which is important during pollen development.
As a component of malate valves, MDH isoforms play a key role in energy homeostasis of plant cells. They catalyze, in a reversible reaction, the interconversion of malate and OAA. Depending on the isoform, NAD+ or NADP+ is used as a coenzyme.77,78 NAD–MDH activities have been detected in microbodies, cytosol, mitochondria and plastids, respectively.79,80 Most of these isoforms were also identified during proteome analyses of mature and developing pollen of various plant species.33,35,37 In addition to the plastid-localized NAD-MDH which is responsible for the removal of NADH to regenerate NAD+ to keep the ATP production via plastidial glycolysis running, 2 mitochondrial MDHs were identified. These MDH isoforms possibly contribute to regenerate NADH (in addition to the TCA cycle) which in turn can be used by Complex I to finally synthesize ATP via respiration (Fig. 2). Furthermore, 2 cytosolic NAD-MDH isoforms could be detected during pollen proteomics. Similar to the NAD-MDH in plastids, cytosolic MDHs could be responsible for the regeneration of NAD+ used by the cytosolic GAPDH (GapC) to synthesize ATP via cytosolic glycolysis (Fig. 2). All these data provide evidence for the important role of MDHs in energy homeostasis during pollen maturation and tube growth. However, until today a detailed analysis of the roles of cytosolic and mitochondrial MDHs in pollen grains and elongating pollen tubes are lacking.
Interestingly, both GapC isoforms could be detected during pollen proteome analyses as well.33,35,37 These isoenzymes were reported to possess additional non-glycolytic activities correlating with their redox-dependent nuclear or cytosolic localization. Therefore, GapCs possibly function as sensor molecules for the detection of H2O2 diffusing out of the chloroplast or mitochondria upon imbalances of the electron transport chains and are part of a redox-dependent retrograde signal transduction network.81-84 Due to fact that gapc1gapc2 double knock-out mutants are fertile, 85 whereas gapcp1gapcp2 double mutants are male sterile, 64 it can be assumed that plastidic glycolysis is more important for pollen maturation and development than is cytosolic glycolysis.
Many basic cellular processes during pollen tube growth are accompanied by various metabolic reactions dependent on NAD+, NADH, NADP+ and NADPH. NAD(P)H exhibit a high energy status and reducing power which drive reductive biosynthetic reactions as well as ATP synthesis.86 The examination of changes in the NAD(P)H content during oscillatory pollen tube growth in lily revealed that NAD(P)H accumulates at the base of the “clear zone” (pollen tube tip) and oscillates with the same period as growth but not in the same phase. Based on these results, it is assumed that an increase in the oxidized state is directly coupled to ATP generation which is used to enable a variety of energy-dependent processes localized in the pollen tube tip.86
In addition, it was recently shown that NAD+ accumulation during pollen maturation regulates the timing of germination onset.87 At low NAD(P)H/NAD(P)+ ratios reactive oxygen species (ROS) generation is prevented by suppressing respiration, and no ATP is generated. A high amount of NAD+ possibly keeps pollen dormant by inhibiting NADH-consuming enzymes.87 It could be observed that an excess amount of NAD+ which accumulates in freshly harvested dry pollen represses the metabolic events controlling pollen tube germination. Upon imbibition the NAD+ concentration decreases and the pollen tube grows.
Although NAD(P)H oxidation is directly coupled to ATP synthesis, it is in parallel involved in the generation of ROS in mitochondria and the plasma membrane. ROS formation itself represents a crucial step during pollen germination. For example, a maximal local growth rate can be achieved by ROS-dependent pollen tube wall-loosening reactions 88, and ROS and reactive nitrogen species (RNS) can additionally serve as signaling molecules in guiding pollen tube growth.4,89,90
Based on these data and the fact that various energy metabolism-related NADH-dependent proteins were identified by comprehensive proteomic studies,33 it is obvious that NAD(P)/H-homeostasis plays an important role during pollen maturation and germination. However, most of the data consider only proteins which are localized in mitochondria (NAD(P)H-dehydrogenases), at the plasma membrane (NAD(P)H oxidase) or which are directly involved in NAD+ biosynthesis (nicotinate/nicotinamide mononucleotide adenyltransferase (NMNAT), nucleotide diphosphate kinase (NDP)).86,87,91,92 But again, it is necessary to consider the importance of the malate valves as well as of cytosolic and plastidial glycolysis in parallel, because these pathways act as powerful systems for balancing the required NAD(P)H/NAD(P)+ and ATP/NAD(P)H ratio which were shown to be very important during pollen maturation and pollen tube elongation.
Impaired pollen tube growth is often accompanied by embryo lethality
Development in general is a high ATP-consuming process. Especially gametophyte, ovule and embryo development need much energy to guarantee successful reproduction. As already mentioned above, pollen maturation and tube growth basically depend on energy generation by mitochondrial respiration and plastidial glycolysis. All of these energy producing pathways are possibly important during ovule and embryo development as well.
In the literature, various examples can be found in which a defect in pollen development and pollen tube elongation is accompanied by abolished ovule development or embryo lethality. Interestingly, most of the knock-out mutants which show defects in pollen development and ovule or embryo development are localized in plastids and are involved in plastidial glycolysis. This fact strengthens the importance of energy generation by plastidial glycolysis during reproductive developmental processes.
For example, the gapcp1/2 double knock-out mutants display a drastic phenotype of arrested root development, dwarfism and sterility. These plants produce shrunken and collapsed pollen grains which are unable to germinate in vitro. In parallel, a delay in ovule development was observed.64,72
In the study of gapcp1/2 double knock-out mutants it could be demonstrated that the arrested root development is due to a deficiency of serine in the roots which is based on a limitation of 3-PGA, the substrate for the phosphorylated pathway of serine biosynthesis (PPSB).64 Based on these observations, the rescue of all identified phenotypes was tested by serine supplementation. Except for the male sterility phenotype, all other phenotypes could be rescued.66 However, the relationship of plastidial glycolysis, especially GapCp1 and GapCp2, and the plastid-localized PPSB during pollen development was further analyzed. Knock-out plants lacking 3-phosphoglycerate dehydrogenase (PGDH; catalyzes the first step during PPSB) or phosphoserine phosphatase (PSP1; catalyzes the last step during PPSB) display arrested microspore development at the polarized stage as well as delayed embryo development, leading to aborted embryos in the early curled cotyledon stage.93-95
Recently, it has been shown that there is a correlation between plNAD-MDH and GapCp expression.66,74 The plNAD-MDH catalyzes the regeneration of NAD+ for GapCp to maintain continuous ATP production via glycolysis. In this context, it is interesting that pollen of heterozygous plnad-mdh knock-out mutants exhibit an abolished pollen tube growth phenotype in vitro. In addition, a homozygous knock-out of plnad-mdh is embryo lethal. In this case, the embryo development stops at the globular stage.66
Another example in which a defect in pollen development is accompanied by abolished ovule development is represented by the ccEe mutant. These knock-out plants possess a reduced number of pollen containing a large portion of underdeveloped, non-vital, non-germinating pollen grains and additionally female gametophyte lethality. In approximately 40% of the ovules, the embryo sac was diminished in size or even absent.63
Besides the already mentioned plastidial enzymes, also mitochondrial proteins could be identified which are involved in pollen vitality and ovule or embryo development, respectively. For instance, GmAOX2b antisense plants exhibit increased rates of pollen abortion in vivo and reduced rates of pollen germination in vitro. In addition, ovule abortion is increased and seed set is reduced.45-46
The genome of A. thaliana contains 3 genes encoding putative Miro GTPases. These proteins contain 2 GTPase domains and 2 calcium-binding motifs. Two of them are localized in mitochondria. A mutation in the miro1 gene is lethal during embryogenesis at the zygote to 4-terminal-cell embryo stage, and it impairs pollen germination and tube growth.96
Although, the genetic roles of nuclear-encoded T3/T7 phage-type RNA polymerases (RPOTs) are largely unknown, it is interesting that knock-out lines of the mitochondrial rpot (rpotm) show retarded pollen tube growth. Also the embryo development is arrested at the globular stage due to an impact on the fusion of the polar nuclei in the embryo sac.97
All of these examples demonstrate that there is a relationship between pollen maturation and development on the one hand, and ovule development and embryo lethality, on the other. All these developmental stages are high energy-consuming steps. Therefore, it is not surprising that such a connection could be observed.
Conclusion
From this review it becomes obvious that energy metabolism during pollen maturation and tube growth is very complex and depends on various mechanisms. Mitochondrial respiration and fermentation are long known to play an important role for energy production during male gametogenesis and pollen tube elongation. However, these data do not provide satisfactory answers to completely explain the energy generation for these developmental processes. For example, it was shown that pollen tube growth in total is much less sensitive to respiratory inhibition than is respiration and that the PDH bypass during fermentation provides only a partial answer concerning the energy generation in growing pollen tubes. Therefore, it could be concluded that further energy-generating pathways contribute additionally to satisfy the energy demand. Glycolysis represents a good candidate due to the ATP generation by substrate phosphorylation existing both, in the cytosol and plastids. Indeed, there are various evidences which definitely demonstrate the important contribution of plastidial glycolysis for energy generation. Many knock-out mutants which lack plastidial glycolytic enzymes exhibit a pollen phenotype or male sterility.
In our model we present various pathways which possibly interplay with each other and therefore contribute to achieve the needed energy demand during pollen maturation and tube growth. Although various data are now available containing information on the impact of mitochondrial respiration, fermentation, plastidial and cytosolic glycolysis as well as the NAD(P)+/NAD(P)H ratio, there is still a high necessity for further investigations concerning the interplay of these pathways of energy metabolism in pollen grains and pollen tubes.
Funding Statement
We greatly acknowledge support from the Deutsche Forschungsgemeinschaft (SFB 944, project P9, R.S.), the government of Lower Saxonia (Lichtenberg fellowship to J.S.) and the Frauenfoerderpool of the University of Osnabrueck (fellowship to J.S.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Cheung AY, Wang H, Wu HM. A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 1995; 82:383-93; PMID:7634328; http://dx.doi.org/ 10.1016/0092-8674(95)90427-1 [DOI] [PubMed] [Google Scholar]
- 2. Dong J, Kim ST, Lord EM. Plantacyanin plays a role in reproduction in Arabidopsis. Plant Physiol 2005; 138:778-9; PMID:15908590; http://dx.doi.org/ 10.1104/pp.105.063388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palanivelu R, Brass L, Edlund AF, Preuss D. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 2003; 114:47-59; PMID:12859897; http://dx.doi.org/ 10.1016/S0092-8674(03)00479-3 [DOI] [PubMed] [Google Scholar]
- 4. Dresselhaus T, Franklin-Tong N. Male-female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol Plant 2013; 6:1018-36; PMID:23571489; http://dx.doi.org/ 10.1093/mp/sst061 [DOI] [PubMed] [Google Scholar]
- 5. Shimizu KK, Okada K. Attractive and repulsive interactions between female and male gametophytes in Arabidopsis pollen tube guidance. Development 2000; 127:4511-8; PMID:11003848 [DOI] [PubMed] [Google Scholar]
- 6. Higashiyama T, Yabe S, Sasaki N, Nishimura Y, Miyagishima S, Kuroiwa H, Kuroiwa T. Pollen tube attraction by the synergid cell. Science 2001; 293:1480-3; PMID:11520985; http://dx.doi.org/ 10.1126/science.1062429 [DOI] [PubMed] [Google Scholar]
- 7. Higashiyama T, Kuroiwa H, Kuroiwa T. Pollen-tube guidance: beacons from the female gametophyte. Curr Opin Plant Biol 2003; 6:36-41; PMID:12495749; http://dx.doi.org/ 10.1016/S1369-5266(02)00010-9 [DOI] [PubMed] [Google Scholar]
- 8. Coimbra S, Jones B, Pereira LG. Arabinogalactan proteins (AGPs) related to pollen tube guidance into the embryo sac in Arabidopsis. Plant Signal Behav 2008; 3:455-6; PMID:19704483; http://dx.doi.org/ 10.4161/psb.3.7.5601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takeuchi H, Higashiyama T. A species-specific cluster of defensin-like genes encodes diffusible pollen tube attractants in Arabidopsis. PLoS Biol 2012; 10: e1001449; http://dx.doi.org/ 10.1371/journal.pbio.1001449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leydon AR, Chaibang A, Johnson MA. Interactions between pollen tube and pistil control pollen tube identity and sperm release in the Arabidopsis female gametophyte. Biochem Soc Trans 2014; 42:340-5; PMID:24646241; http://dx.doi.org/ 10.1042/BST20130223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McCormick S. Control of male gametophyte development. Plant Cell 2004; 16:142-53; http://dx.doi.org/ 10.1105/tpc.016659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Franklin-Tong VE. Signaling and the modulation of pollen tube growth. Plant Cell 1999; 11:727-38; PMID:10213789; http://dx.doi.org/ 10.1105/tpc.11.4.727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barnabas B, Fridvaiszky L. Adhesion and germination of differently treated maize pollen grains on the stigma. Acta Bot Hungar 1984; 30:329-32 [Google Scholar]
- 14. Hepler PK, Vidali L, Cheung AY. Polarized cell growth in higher plants. Annu Rev Cell Dev Biol 2001; 17:159-87; PMID:11687487; http://dx.doi.org/ 10.1146/annurev.cellbio.17.1.159 [DOI] [PubMed] [Google Scholar]
- 15. Stone LM, Seaton KA, Kuo J, McComb JA. Fast pollen tube growth in Conospermum species. Ann Bot 2004; 93:369-78; PMID:14980970; http://dx.doi.org/ 10.1093/aob/mch050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howden R, Park SK, Moore JM, Orme J, Grossniklaus U, Twell D. Selection of T-DNA-tagged male and female gametophytic mutants by segregation distortion in Arabidopsis. Genetics 1998; 149:621-31; PMID:9611178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee SLJ, Warmke HE. Organelle size and number in fertile and T-cytoplasmic male-sterile corn. Am J Bot 1979; 66:141-8; http://dx.doi.org/ 10.2307/2442516 [DOI] [Google Scholar]
- 18. Dickinson DB. Germination of lily pollen: Respiration and tube growth. Science 1965; 150:1818-9; PMID:17841976; http://dx.doi.org/ 10.1126/science.150.3705.1818 [DOI] [PubMed] [Google Scholar]
- 19. Taylor LP, Hepler PK. Pollen germination and tube growth. Annu Rev Plant Physiol Plant Mol Biol 1997; 48:461-91; PMID:15012271; http://dx.doi.org/ 10.1146/annurev.arplant.48.1.461 [DOI] [PubMed] [Google Scholar]
- 20. Tadege M, Kuhlemeier C. Aerobic fermentation during tobacco pollen development. Plant Mol Biol 1997; 35:343-54; PMID:9349258; http://dx.doi.org/ 10.1023/A:1005837112653 [DOI] [PubMed] [Google Scholar]
- 21. Brewbaker JL. The distribution and phylogenetic significance of binucleate and trinucleate pollen grains in the angiosperms. Am J Bot 1967; 54:1069-83; http://dx.doi.org/ 10.2307/2440530 [DOI] [Google Scholar]
- 22. Hoekstra FA, Bruinsma J. Respiration and vitality of binucleate and trinucleate pollen. Physiol Planta 1975; 34:221-5; http://dx.doi.org/ 10.1111/j.1399-3054.1975.tb03825.x [DOI] [Google Scholar]
- 23. Colaço R, Moreno N, Feijó JA. On the fast lane: mitochondria structure, dynamics and function in growing pollen tubes. J Microsc 2012; 247:106-18; http://dx.doi.org/ 10.1111/j.1365-2818.2012.03628.x [DOI] [PubMed] [Google Scholar]
- 24. Feijó JA, Moreno N. Imaging plant cells by two-photon excitation. Protoplasma 2004; 223:1-32; http://dx.doi.org/ 10.1007/s00709-003-0026-2 [DOI] [PubMed] [Google Scholar]
- 25. Lovy-Wheeler A, Cárdenas L, Kunkel JG, Hepler PK. Differential organelle movement on the actin cytoskeleton in lily pollen tubes. Cell Motil Cytoskeleton 2007; 64:217-32; PMID:17245769; http://dx.doi.org/ 10.1002/cm.20181 [DOI] [PubMed] [Google Scholar]
- 26. Matsushima R, Hamamura Y, Higashiyama T, Arimura S, Sodmergen , Tsutsumi N, Sakamoto W. Mitochondrial dynamics in plant male gametophyte visualized by fluorescent live imaging. Plant Cell Physiol 2008; 49:1074-83; PMID:18522988; http://dx.doi.org/ 10.1093/pcp/pcn084 [DOI] [PubMed] [Google Scholar]
- 27. Parton RM, Fischer-Parton S, Watahiki MK, Trewavas AJ. Dynamics of the apical vesicle accumulation and the rate of growth are related in individual pollen tubes. J Cell Sci 2001; 114:2685-95; PMID:11683395 [DOI] [PubMed] [Google Scholar]
- 28. Vidali L, McKenna ST, Hepler PK. Actin polymerization is essential for pollen tube growth. Mol Biol Cell 2001; 12:2534-45; PMID:11514633; http://dx.doi.org/ 10.1091/mbc.12.8.2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Certal AC, Almeida RB, Carvalho LM, Wong E, Moreno N, Michard E, Carneiro J, Rodriguéz-Léon J, Wu HM, Cheung AY, Feijó JA. Exclusion of a proton ATPase from the apical membrane is associated with cell polarity and tip growth in Nicotiana tabacum pollen tubes. Plant Cell 2008; 20:614-34; PMID:18364468; http://dx.doi.org/ 10.1105/tpc.106.047423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schiøtt M, Romanowsky SM, Baekgaard L, Jakobsen MK, Palmgren MG, Harper JF. A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc Natl Acad Sci USA 2004; 101:9502-7; http://dx.doi.org/ 10.1073/pnas.0401542101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Honys D, Twell D. Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiol 2003; 132:640-52; PMID:12805594; http://dx.doi.org/ 10.1104/pp.103.020925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Honys D, Twell D. Transcriptome analysis of haploid male gametophyte development in Arabidopsis. Genome Biol 2004; 5: R85; PMID:15535861; http://dx.doi.org/ 10.1186/gb-2004-5-11-r85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holmes-Davis R, Tanaka CK, Vensel WH, Hurkman WJ, McCormick S. Proteome mapping of mature pollen of Arabidopsis thaliana. Proteomics 2005; 5:4864-84; PMID:16247729; http://dx.doi.org/ 10.1002/pmic.200402011 [DOI] [PubMed] [Google Scholar]
- 34. Becker JD, Feijó JA. How many genes are needed to make a pollen tube? Lessons from transcriptomics. Ann Bot 2007; 100:1117-23; PMID:17951360; http://dx.doi.org/ 10.1093/aob/mcm208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sheoran IS, Ross AR, Olson DJ, Sawhney VK. Proteomic analysis of tomato (Lycopersicon esculentum) pollen. J Exp Bot 2007; 58:3525-35; PMID:17921476; http://dx.doi.org/ 10.1093/jxb/erm199 [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Zhang WZ, Song LF, Zou JJ, Su Z, Wu WH. Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in Arabidopsis. Plant Physiol 2008; 148:1201-11; PMID:18775970; http://dx.doi.org/ 10.1104/pp.108.126375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zou J, Song L, Zhang W, Wang Y, Ruan S, Wu WH. Comparative proteomic analysis of Arabidopsis mature pollen and germinated pollen. J Integr Plant Biol 2009; 51:438-55; PMID:19508356; http://dx.doi.org/ 10.1111/j.1744-7909.2009.00823.x [DOI] [PubMed] [Google Scholar]
- 38. Rafinska K, Zienkiewicz K, Bednarska E. Pollen transcriptome and proteome: molecular and functional analysis. Adv Cell Biol 2010; 2:29-57; http://dx.doi.org/ 10.2478/v10052-010-0003-9 [DOI] [Google Scholar]
- 39. Fujii S, Komatsu S, Toriyama K. Retrograde regulation of nuclear gene expression in CW-CMS of rice. Plant Mol Biol 2007; 63:405-17; PMID:17086445; http://dx.doi.org/ 10.1007/s11103-006-9097-8 [DOI] [PubMed] [Google Scholar]
- 40. Fujii S, Toriyama K. DCW11, down-regulated gene 11 in CW-type cytoplasmic male sterile rice, encoding mitochondrial protein phosphatase 2c is related to cytoplasmic male sterility. Plant Cell Physiol 2008; 49:633-40; PMID:18308761; http://dx.doi.org/ 10.1093/pcp/pcn036 [DOI] [PubMed] [Google Scholar]
- 41. Rounds CM, Winship LJ, Hepler PK. Pollen tube energetics: respiration, fermentation and the race to the ovule. AoB Plants 2011; 2011:plr019; PMID:22476489; http://dx.doi.org/ 10.1093/aobpla/plr019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Johns C, Nickels R, McIntosh L, Mackenzie S. The expression of alternative oxidase and alternative respiratory capacity in cytoplasmic male sterile common bean. Sex Plant Reprod 1993; 6:257-65; http://dx.doi.org/ 10.1007/BF00231903 [DOI] [Google Scholar]
- 43. Conley CA, Hanson MR. Tissue-specific protein expression in plant mitochondria. Plant Cell 1994; 6:85-91; PMID:12244222; http://dx.doi.org/ 10.1105/tpc.6.1.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kitashiba H, Kitazawa E, Kishitani S, Toriyama K. Partial male sterility in transgenic tobacco carrying an antisense gene for alternative oxidase under the control of a tapetum-specific promoter. Mol Breed 1999; 5:209-18; http://dx.doi.org/ 10.1023/A:1009606601521 [DOI] [Google Scholar]
- 45. Chai TT, Simmonds D, Day DA, Colmer TD, Finnegan PM. Photosynthetic performance and fertility are repressed in GmAOX2b antisense soybean. Plant Physiol 2010; 152:1638-49; PMID:20097793; http://dx.doi.org/ 10.1104/pp.109.149294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chai TT, Colmer TD, Finnegan PM. Alternative oxidase, a determinant of plant gametophyte fitness and fecundity. Plant Signal Behav 2010; 5:604-6; PMID:20404537; http://dx.doi.org/ 10.4161/psb.11502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rounds CM, Hepler PK, Fuller SJ, Winship LJ. Oscillatory growth in lily pollen tubes does not require aerobic energy metabolism. Plant Physiol 2010; 152:736-46; PMID:20007440; http://dx.doi.org/ 10.1104/pp.109.150896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Obermeyer G, Fragner L, Lang V, Weckwerth W. Dynamic adaption of metabolic pathways during germination and growth of lily pollen tubes after inhibition of the electron transport chain. Plant Physiol 2013; 162:1822-33; PMID:23660836; http://dx.doi.org/ 10.1104/pp.113.219857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Blasiak J, Mulcahy DL, Musgrave M. Oxytropism: a new twist in pollen tube orientation. Planta 2001; 213:318-22; PMID:11469598; http://dx.doi.org/ 10.1007/s004250000495 [DOI] [PubMed] [Google Scholar]
- 50. Linskens HF, Schrauwen J. Measurement of oxygen tension changes in the style during pollen tube growth. Planta 1966; 71:98-106; PMID:24553992; http://dx.doi.org/ 10.1007/BF00384646 [DOI] [PubMed] [Google Scholar]
- 51. Bucher M, Brander KA, Sbicego S, Mandel T, Kuhlemeier C. Aerobic fermentation in tobacco pollen. Plant Mol Biol 1995; 28:739-50; PMID:7647304; http://dx.doi.org/ 10.1007/BF00021197 [DOI] [PubMed] [Google Scholar]
- 52. Tadege M, Brändle R, Kuhlemeier C. Anoxia tolerance in tobacco roots: effect of overexpression of pyruvate decarboxylase. Plant J 1998; 14:327-35; http://dx.doi.org/ 10.1046/j.1365-313X.1998.00130.x [DOI] [Google Scholar]
- 53. Freeling M, Bennett DC. Maize Adh1. Annu Rev Genet 1985; 19:297-323; PMID:3002240; http://dx.doi.org/ 10.1146/annurev.ge.19.120185.001501 [DOI] [PubMed] [Google Scholar]
- 54. Tadege M, Dupuis I, Kuhlemeier C. Ethanolic fermentation: new functions for an old pathway. Trends Plant Sci 1999; 4:320-5; PMID:10431222; http://dx.doi.org/ 10.1016/S1360-1385(99)01450-8 [DOI] [PubMed] [Google Scholar]
- 55. Mellema S, Eichenberger W, Rawyler A, Suter M, Tadege M, Kuhlemeier C. The ethanolic fermentation pathway supports respiration and lipid biosynthesis in tobacco pollen. Plant J 2002; 30:329-36; PMID:12000680; http://dx.doi.org/ 10.1046/j.1365-313X.2002.01293.x [DOI] [PubMed] [Google Scholar]
- 56. Gass N, Glagotskaia T, Mellema S, Stuurman J, Barone M, Mandel T, Roessner-Tunali U, Kuhlemeier C. Pyruvate decarboxylase provides growing pollen tubes with a competitive advantage in petunia. Plant Cell 2005; 17:2355-68; PMID:15994907; http://dx.doi.org/ 10.1105/tpc.105.033290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tang LY, Nagata N, Matsushima R, Chen Y, Yoshioka Y, Sakamoto W. Visualization of plastids in pollen grains: involvement of FtsZ1 in pollen plastid division. Plant Cell Physiol 2009; 50:904-8; PMID:19282372; http://dx.doi.org/ 10.1093/pcp/pcp042 [DOI] [PubMed] [Google Scholar]
- 58. Fujiwara MT, Hashimoto H, Kazama Y, Hirano T, Yoshioka Y, Aoki S, Sato N, Itoh RD, Abe T. Dynamic morphologies of pollen plastids visualised by vegetative-specific FtsZ1-GFP in Arabidopsis thaliana. Protoplasma 2010; 242:19-33; PMID:20195657; http://dx.doi.org/ 10.1007/s00709-010-0119-7 [DOI] [PubMed] [Google Scholar]
- 59. Fujiwara MT, Yoshioka Y, Hirano T, Kazama Y, Abe T, Hayashi K, Itoh RD. Visualization of plastid movement in the pollen tube of Arabidopsis thaliana. Plant Signal Behav 2012; 7:34-7; PMID:22301964; http://dx.doi.org/ 10.4161/psb.7.1.18484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Van Aelst AC, Pierson ES, Van Went JL, Cresti M. Ultrastructural changes of Arabidopsis thaliana pollen during final maturation and rehydration. Zygote 1993; 1:173-9; PMID:8081813 [DOI] [PubMed] [Google Scholar]
- 61. Nagata N, Saito C, Sakai A, Kuroiwa H, Kuroiwa T. The selective increase or decrease of organellar DNA in generative cells just after pollen mitosis I controls cytoplasmic inheritance. Planta 1999; 209:53-65; PMID:10467031; http://dx.doi.org/ 10.1007/s004250050606 [DOI] [PubMed] [Google Scholar]
- 62. Kuang A, Musgrave ME. Dynamics of vegetative cytoplasm during generative cell formation and pollen maturation in Arabidopsis thaliana. Protoplasma 1996; 194:81-90; PMID:11540605; http://dx.doi.org/ 10.1007/BF01273170 [DOI] [PubMed] [Google Scholar]
- 63. Prabhakar V, Löttgert T, Geimer S, Dörmann P, Krüger S, Vijayakumar V, Schreiber L, Göbel C, Feussner K, Feussner I, Marin K, Staehr P, Bell K, Flügge UI, Häusler RE. Phosphoenolpyruvate provision to plastids is essential for gametophyte and sporophyte development in Arabidopsis thaliana. Plant Cell 2010; 22:2594-617; PMID:20798327; http://dx.doi.org/ 10.1105/tpc.109.073171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Muñoz-Bertomeu J, Cascales-Miñana B, Irles-Segura A, Mateu I, Nunes-Nesi A, Fernie AR, Segura J, Ros R. The plastidial glyceraldehyde-3-phosphate dehydrogenase is critical for viable pollen development in Arabidopsis. Plant Physiol 2010; 152:1830-41; http://dx.doi.org/ 10.1104/pp.109.150458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhao Z, Assmann SM. The glycolytic enzyme, phosphoglycerate mutase, has critical roles in stomatal movement, vegetative growth, and pollen production in Arabidopsis thaliana. J Exp Bot 2011; 62:5179-89; PMID:21813794; http://dx.doi.org/ 10.1093/jxb/err223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Selinski J, König N, Wellmeyer B, Hanke GT, Linke V, Neuhaus HE, Scheibe R. The plastid-localized NAD-dependent malate dehydrogenase is crucial for energy homeostasis in developing Arabidopsis thaliana seeds. Mol Plant 2014; 7:170-86; PMID:24198233; http://dx.doi.org/ 10.1093/mp/sst151 [DOI] [PubMed] [Google Scholar]
- 67. Datta R, Chamusco KC, Chourey PS. Starch biosynthesis during pollen maturation is associated with altered patterns of gene expression in maize. Plant Physiol 2002; 130:1645-56; PMID:12481048; http://dx.doi.org/ 10.1104/pp.006908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wen LY, Chase CD. Mitochondrial gene expression in developing male gametophytes of male-fertile and S male-sterile maize. Sex Plant Reprod 1999; 11:323-30; http://dx.doi.org/ 10.1007/s004970050159 [DOI] [Google Scholar]
- 69. Dickinson DB. Rapid starch synthesis associated with increased respiration in germinating lily pollen. Plant Physiol 1968; 43:1-8; PMID:16656725; http://dx.doi.org/ 10.1104/pp.43.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Baalmann E, Backhausen JE, Rak C, Vetter S, Scheibe R. Reductive modification and nonreductive activation of purified spinach chloroplast NADP-dependent glyceraldehyde-3-phosphate dehydrogenase. Arch Biochem Biophys 1995; 324:201-8; PMID:8554310; http://dx.doi.org/ 10.1006/abbi.1995.0031 [DOI] [PubMed] [Google Scholar]
- 71. Backhausen JE, Vetter S, Baalmann E, Kitzmann C, Scheibe R. NAD-dependent malate dehydrogenase and glyceraldehyde 3-phosphate dehydrogenase isoenzymes play an important role in dark metabolism of various plastid types. Planta 1998; 205:359-66; http://dx.doi.org/ 10.1007/s004250050331 [DOI] [Google Scholar]
- 72. Muñoz-Bertomeu J, Cascales-Miñana B, Mulet JM, Baroja-Fernández E, Pozueta Romero J, Kuhn JM, Segura J, Ros R. Plastidial glyceraldehyde-3-phosphate dehydrogenase deficiency leads to altered root development and affects the sugar and amino acid balance in Arabidopsis. Plant Physiol 2009; 151:541-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Scheibe R. Malate valves to balance cellular energy supply. Physiol Plant 2004; 120:21-6; PMID:15032873; http://dx.doi.org/ 10.1111/j.0031-9317.2004.0222.x [DOI] [PubMed] [Google Scholar]
- 74. Selinski J, Scheibe R. Lack of malate valve capacities lead to improved N-assimilation and growth in transgenic A. thaliana plants. Plant Signal Behav 2014; 9: e29057; PMID:24801518; http://dx.doi.org/ 10.4161/psb.29057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Prabhakar V, Löttgert T, Gigolashvili T, Bell K, Flügge UI, Häusler RE. Molecular and functional characterization of the plastid-localized phosphoenolpyruvate enolase (ENO1) from Arabidopsis thaliana. FEBS Lett 2009; 583:983-91; PMID:19223001; http://dx.doi.org/ 10.1016/j.febslet.2009.02.017 [DOI] [PubMed] [Google Scholar]
- 76. Reiser J, Linka N, Lemke L, Jeblick W, Neuhaus HE. Molecular physiological analysis of the two plastidic ATP/ADP transporters from Arabidopsis. Plant Physiol 2004; 136:3524-36; PMID:15516503; http://dx.doi.org/ 10.1104/pp.104.049502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ocheretina O, Scheibe R. Cloning and sequence analysis of cDNAs encoding plant cytosolic malate dehydrogenase. Gene 1997; 199:145-8; PMID:9358050; http://dx.doi.org/ 10.1016/S0378-1119(97)00361-2 [DOI] [PubMed] [Google Scholar]
- 78. Scheibe R. Malate valves to balance cellular energy supply. Physiol Plant 2004; 120:21-6; PMID:15032873; http://dx.doi.org/ 10.1111/j.0031-9317.2004.0222.x [DOI] [PubMed] [Google Scholar]
- 79. Berkemeyer M, Scheibe R, Ocheretina O. A novel, non-redox-regulated NAD-dependent malate dehydrogenase from chloroplasts of Arabidopsis thaliana L. J Biol Chem 1998; 273:27927-33; PMID:9774405; http://dx.doi.org/ 10.1074/jbc.273.43.27927 [DOI] [PubMed] [Google Scholar]
- 80. Gietl C. Malate dehydrogenase isoenzymes: cellular locations and role in the flow of metabolites between the cytoplasm and cell organelles. Biochim Biophys Acta 1992; 1100:217-34; PMID:1610875; http://dx.doi.org/ 10.1016/0167-4838(92)90476-T [DOI] [PubMed] [Google Scholar]
- 81. Holtgrefe S, Gohlke J, Starmann J, Druce S, Klocke S, Altmann B, Wojtera J, Lindermayr C, Scheibe R. Regulation of plant cytosolic glyceraldehyde 3-phosphate dehydrogenase isoforms by thiol modifications. Physiol Plant 2008; 133:211-28; PMID:18298409; http://dx.doi.org/ 10.1111/j.1399-3054.2008.01066.x [DOI] [PubMed] [Google Scholar]
- 82. Wojtera-Kwiczor J, Groß F, Leffers HM, Kang M, Schneider M, Scheibe R. Transfer of a redox-signal through the cytosol by redox-dependent microcompartmentation of glycolytic enzymes at mitochondria and actin cytoskeleton. Front Plant Sci 2013; 3:284; PMID:23316205; http://dx.doi.org/ 10.3389/fpls.2012.00284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zachgo S, Hanke GT, Scheibe R. Plant cell microcompartments: a redox-signaling perspective. Biol Chem 2013; 394:203-16; PMID:23241667; http://dx.doi.org/ 10.1515/hsz-2012-0284 [DOI] [PubMed] [Google Scholar]
- 84. Hancock J, Desikan R, Harrison J, Bright J, Hooley R, Neill S. Doing the unexpected: proteins involved in hydrogen peroxide perception. J Exp Bot 2006; 57:1711-8; PMID:16595577; http://dx.doi.org/ 10.1093/jxb/erj180 [DOI] [PubMed] [Google Scholar]
- 85. Guo L, Devaiah SP, Narasimhan R, Pan X, Zhang Y, Zhang W, Wang X. Cytosolic glyceraldehyde-3-phosphate dehydrogenases interact with phospholipase Dδ to transduce hydrogen peroxide signals in the Arabidopsis response to stress. Plant Cell 2012; 24:2200-12; PMID:22589465; http://dx.doi.org/ 10.1105/tpc.111.094946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cárdenas L, McKenna ST, Kunkel JG, Hepler PK. NAD(P)H oscillates in pollen tubes and is correlated with tip growth. Plant Physiol 2006; 142:1460-68; http://dx.doi.org/ 10.1104/pp.106.087882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hashida SN, Takahashi H, Takahara K, Kawai-Yamada M, Kitazaki K, Shoji K, Goto F, Yoshihara T, Uchimiya H. NAD+ accumulation during pollen maturation in Arabidopsis regulating onset of germination. Mol Plant 2013; 6:216-25; PMID:22907882; http://dx.doi.org/ 10.1093/mp/sss071 [DOI] [PubMed] [Google Scholar]
- 88. Liszkay A, van der Zalm E, Schopfer P. Production of reactive oxygen intermediates (O2.-, H2O2, and.OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol 2004; 136:3114-23; PMID:15466236; http://dx.doi.org/ 10.1104/pp.104.044784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. McInnis SM, Desikan R, Hancock JT, Hiscock SJ. Production of reactive oxygen species and reactive nitrogen species by angiosperm stigmas and pollen: potential signalling crosstalk? New Phytol 2006; 172:221-8; PMID:16995910; http://dx.doi.org/ 10.1111/j.1469-8137.2006.01875.x [DOI] [PubMed] [Google Scholar]
- 90. Hiscock SJ, Bright J, McInnis SM, Desikan R, Hancock JT. Signaling on the stigma: potential new roles for ROS and NO in plant cell signaling. Plant Signal Behav 2007; 2:23-4; PMID:19704802; http://dx.doi.org/ 10.4161/psb.2.1.3644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Potocký M, Jones MA, Bezvoda R, Smirnoff N, Zárský V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol 2007; 174:742-51; PMID:17504458; http://dx.doi.org/ 10.1111/j.1469-8137.2007.02042.x [DOI] [PubMed] [Google Scholar]
- 92. Lassig R, Gutermuth T, Bey TD, Konrad KR, Romeis T. Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant J 2014; 78:94-106; PMID:24506280; http://dx.doi.org/ 10.1111/tpj.12452 [DOI] [PubMed] [Google Scholar]
- 93. Toujani W, Muñoz-Bertomeu J, Flores-Tornero M, Rosa-Téllez S, Anoman AD, Alseekh S, Fernie AR, Ros R. Functional characterization of the plastidial 3-phosphoglycerate dehydrogenase family in Arabidopsis. Plant Physiol 2013; 163:1164-1178; PMID:24058165; http://dx.doi.org/ 10.1104/pp.113.226720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cascales-Miñana B, Muñoz-Bertomeu J, Flores-Tornero M, Anoman AD, Pertusa J, Alaiz M, Osorio S, Fernie AR, Segura J, Ros R. The phosphorylated pathway of serine biosynthesis is essential both for male gametophyte and embryo development and for root growth in Arabidopsis. Plant Cell 2013; 25:2084-101; http://dx.doi.org/ 10.1105/tpc.113.112359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Muñoz-Bertomeu J, Anoman A, Flores-Tornero M, Toujani W, Rosa-Téllez S, Fernie AR, Roje S, Segura J, Ros R. The essential role of the phosphorylated pathway of serine biosynthesis in Arabidopsis. Plant Signal Behav 2013; 8: e27104; http://dx.doi.org/ 10.4161/psb.27104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yamaoka S, Leaver CJ. EMB2473/MIRO1, an Arabidopsis Miro GTPase, is required for embryogenesis and influences mitochondrial morphology in pollen. Plant Cell 2008; 20:589-601; PMID:18344283; http://dx.doi.org/ 10.1105/tpc.107.055756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tan XY, Liu XL, Wang W, Jia DJ, Chen LQ, Zhang XQ, Ye D. Mutations in the Arabidopsis nuclear-encoded mitochondrial phage-type RNA polymerase gene RPOTm led to defects in pollen tube growth, female gametogenesis and embryogenesis. Plant Cell Physiol 2010; 51:635-49; PMID:20231244; http://dx.doi.org/ 10.1093/pcp/pcq029 [DOI] [PubMed] [Google Scholar]