Figure 2.
WIP Deficiency Affects B Cell Homing and Chemotaxis
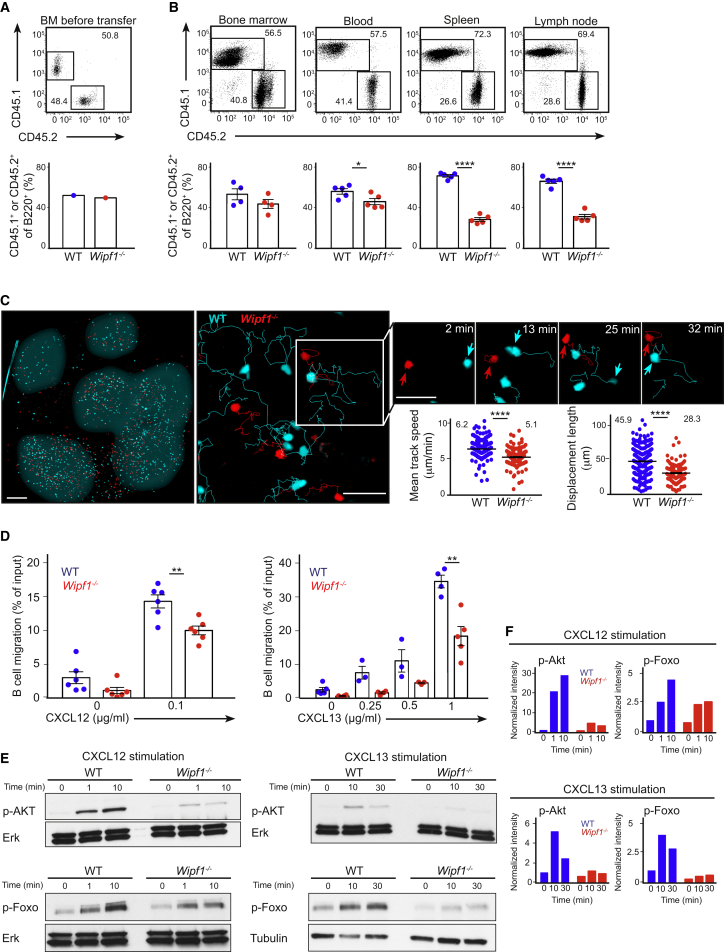
(A) Flow cytometric analysis of BM mixture (B220+CD45.1+ WT and B220+CD45.2+Wipf1−/− BM in a mixture of 1:1) before transfer into irradiated recipients.
(B) Flow cytometric analysis of WT cells (B220+CD45.1+) and Wipf1−/− cells (B220+CD45.2+) in indicated organs of mixed BM chimeras (upper row). Graphs (±SEM) indicate the percentage of B220+CD45.1+ WT and B220+CD45.2+Wipf1−/− cells in respective organs. Data are representative of three independent experiments (n ≥ 4 mice).
(C) Representative two-photon microscopy images 24 hr after adoptive transfer of labeled WT (cyan) and Wipf1−/− (red) B cells. Left image is a whole fixed popliteal lymph node (scale bar, 150 μm). B cell follicles are highlighted in gray for illustrative purposes. Middle and right images are examples of the visualization (including representative tracks) of homeostatic movement (scale bars, 30 μm). Smaller images indicate two representative cells, highlighted by arrows at different times. Graphs (±SEM) on the bottom right indicate the quantification of the average speed over the span of the movie and the mean displacement length. Data are representative of three independent experiments.
(D) Chemotactic response of purified WT and Wipf1−/− B cells to CXCL12 (left graph) or CXCL13 (right graph) using transwell plates. Graphs (±SEM) show percentages of migrating cells normalized to the starting input number of B cells set to 100%. Data are pooled from four independent experiments.
(E) Immunoblot of splenic WT or Wipf1−/− B cells treated with CXCL12 or CXCL13 and probed with antibodies as indicated.
(F) Quantifications of intensity of proteins normalized by densitometry to total Erk or tubulin and to the signal in unstimulated WT cells at t = 0. Data are representative of at least three independent experiments.