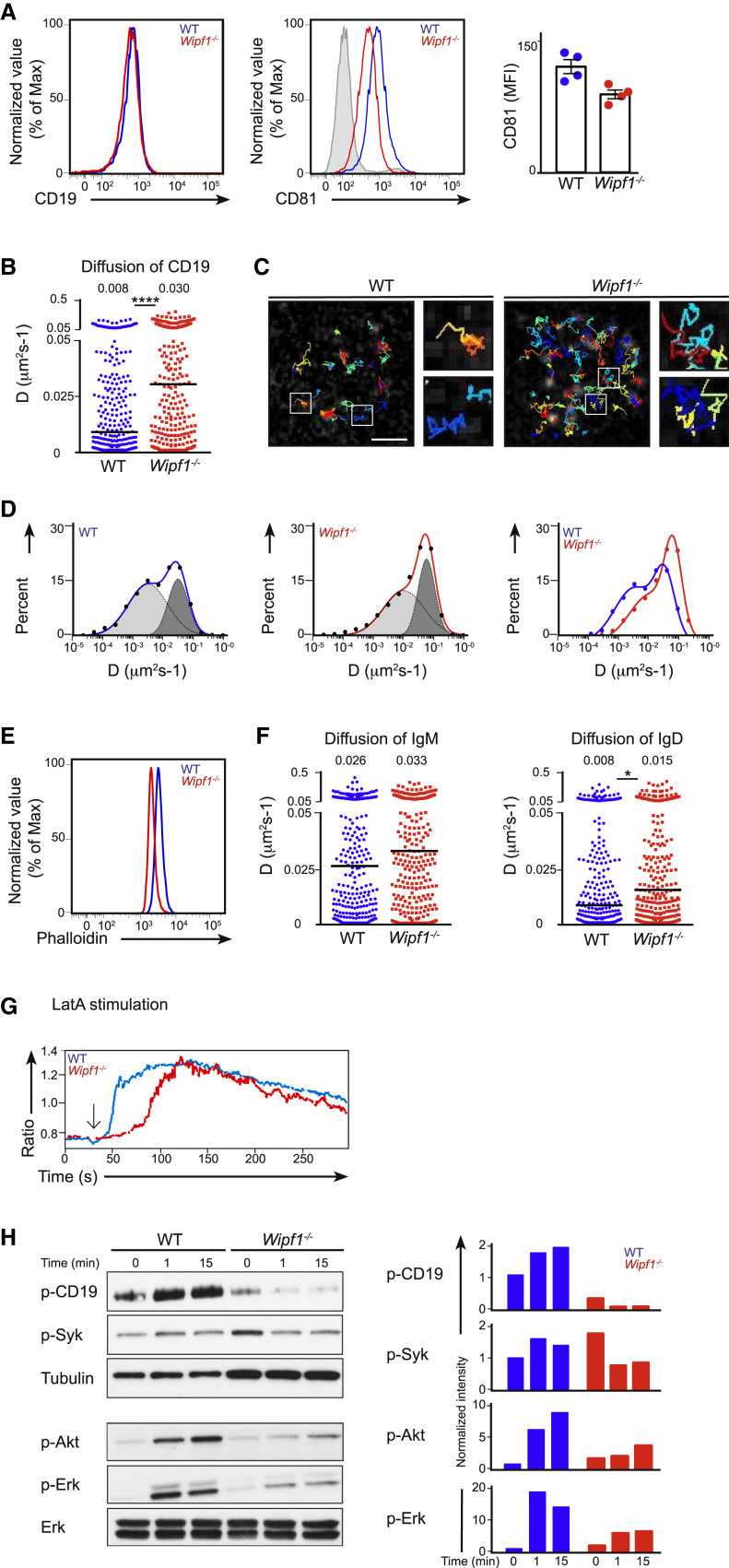
Figure 6.
WIP Regulates BCR and CD19 Diffusion on the B Cell Surface
(A) Flow cytometric analysis of CD19 and CD81 on the surface of WT (blue line) or Wipf1−/− (red line) B cells. Grey shaded histogram indicates the isotype control. Graph (mean ± SEM) on the right shows the MFI of CD81 expression on splenic B cells of 4 WT and 4 Wipf1−/− mice.
(B) Single-particle tracking (SPT) of CD19 on WT or Wipf1−/− B cells settled on nonstimulatory coverslips. Shown is the diffusion coefficient (D) in μm2/s. The median of 300 representative cells is given as value and indicated by black bars. 1,000–2,000 tracks were analyzed with a minimum of 20 cells from 3 experiments.
(C) Trajectories of CD19 in WT (left) and Wipf1−/− cells (right) showing diffusion of single particles over 10 s and magnified regions from white rectangles. Scale bar, 2 μm.
(D) Analysis of the CD19 diffusion coefficient plotted in a logarithmic histogram and fitted to two distinct populations exhibiting slower (light gray) and faster (dark gray) diffusion on WT (left graph), Wipf1−/− (middle graph) and a comparison of the CD19 diffusion on WT and Wipf1−/− B cells (right graph).
(E) Flow cytometric analysis of F-actin by intracellular phalloidin staining of resting WT (blue line) or Wipf1−/− (red line) B cells.
(F) SPT of IgM and IgD on WT or Wipf1−/− B cells settled on nonstimulatory coverslips. Shown is the diffusion coefficient (D) in μm2/s. 1,000–2,000 tracks were analyzed with a minimum of 20 cells from 2 experiments. The median of 300 representative cells is given as value and indicated by black bars.
(G) Ratiometric intracellular Ca2+ flux of WT and Wipf1−/− B cells, stimulated with Latranculin A and analyzed by flow cytometry. Results are presented as fluorescent emission at 450 nm relative to that at 530 nm. Data are representative of three independent experiments.
(H) Immunoblot of splenic WT or Wipf1−/− B cells stimulated with Latrunculin A and probed with antibodies as indicated. Quantifications of intensity of proteins normalized by densitometry to Erk or tubulin and to the signal in unstimulated WT cells at t = 0.