Figure 2.
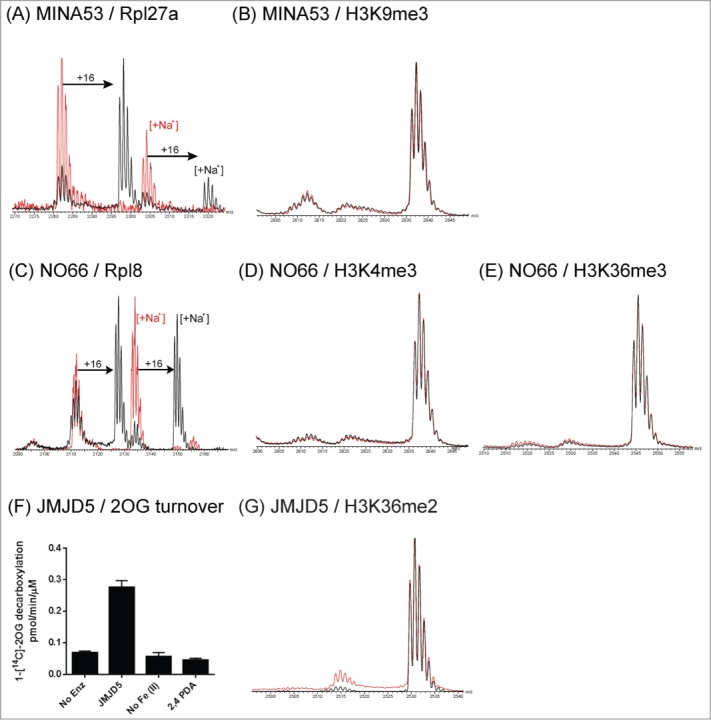
JmjC Oxygenases MINA53, NO66 and JMJD5 do not catalyze demethylation of histone peptides. In addition to putative demethylation activities, MINA53 and NO66 have been characterized as hydroxylases acting on ribosomal proteins Rpl27a and Rpl8 respectively. Hydroxylation activities were observed for MINA53 and NO66, acting on Rpl27a and Rpl8 peptide fragments respectively (A and C); no demethylation was observed with methylated histone peptides (B, D and E). Prime-substrate uncoupled turnover of 2OG by JMJD5 (residues 1–416) was observed in a [14C]-labeled 2OG assay, which was dependent on the presence of iron(II) and inhibited by the broad-spectrum 2OG oxygenase inhibitor 2,4-pyridinedicarboxylic acid (2,4 PDA) (F). However, demethylation of an H3K36me2 histone peptide was not observed (G). Control reactions without added protein are in red.
