Figure 2.
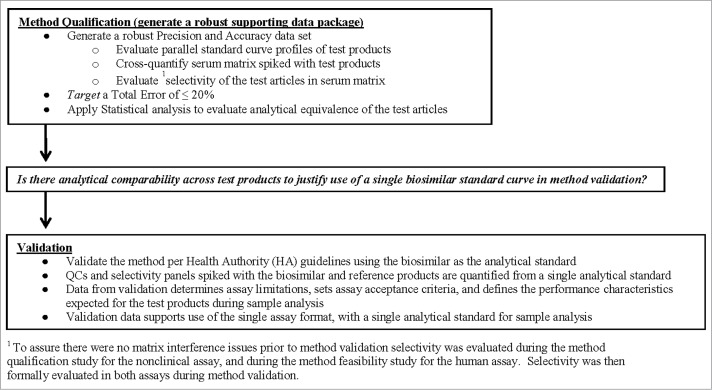
Outline of the testing paradigm applied to the single PK assay approach. The testing began with a robust method qualification study where statistical analysis of the data was used to determine if the test products were analytically equivalent within the method. If results from method qualification demonstrated the test products were comparable, then the data served to justify use of a single assay calibrator for quantification of the biosimilar and reference products during method validation and sample analysis.
