Figure 6.
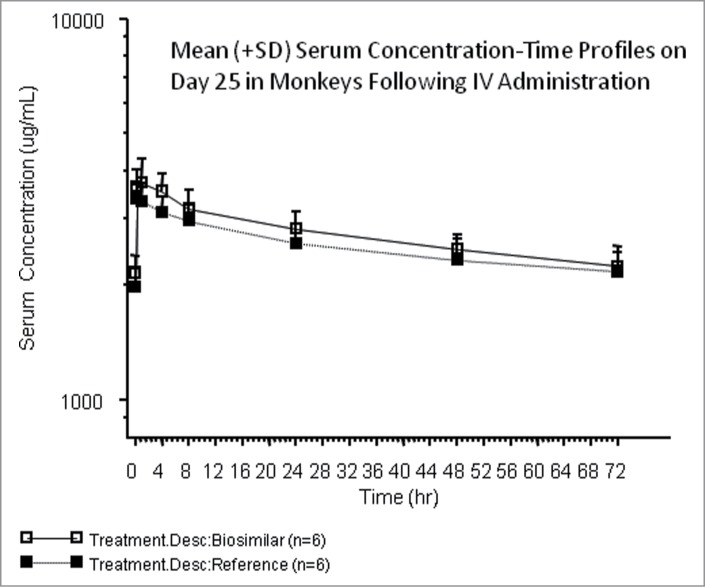
The nonclinical method was implemented to support the sample analysis phase of the toxicology study. Study performance data was consistent with performance data observed during validation (Table 1). As displayed in Figure 6, results from the toxicokinetic study showed that the mean concentration-time profiles in-vivo were similar between the biosimilar and FDA-licensed reference groups following administration of the test products weekly.
