Figure 1.
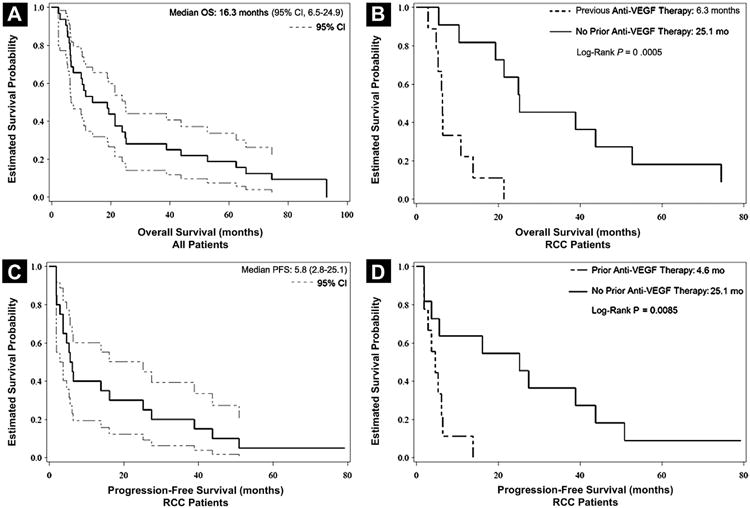
Survival and Progression-Free Survival (PFS) in Patients Treated With Vatalanib and Everolimus. (A) Overall Survival (OS) in all Patients (n = 32). Dashed Lines Represent 95% Confidence Intervals (CIs). Median Survival Time is 16.3 Months (95% CI, 6.5-24.9). (B) OS Comparing Patients With Renal Cell Carcinoma (RCC) in the Dose-Expansion Cohort who had Received Previous Vascular Endothelial Growth Factor (VEGF) Therapy (n = 10) vs. Those who had Received no Previous VEGF Therapy (n = 10). Median OS in Patients With Previous VEGF Therapy was 6.3 Months vs. 25.1 Months in Patients With no Previous VEGF Therapy (log-Rank P = .0005). (C) PFS in Patients With RCC Treated at the Maximum Tolerated Dose (MTD) (n = 20). Dashed Lines Represent 95% CI. Median PFS was 5.8 Months (95% CI, 2.8-25.1). (D) PFS Comparing Patients With RCC in the Dose-Expansion Cohort who had Received Previous VEGF Therapy (n = 10) and Those who had Received no Previous VEGF Therapy (n = 10). Median PFS in Patients who had Received Previous VEGF Therapy is 4.6 Months vs. 25.1 Months in Patients who had not Received Previous VEGF Therapy (log-Rank P = .0085)
