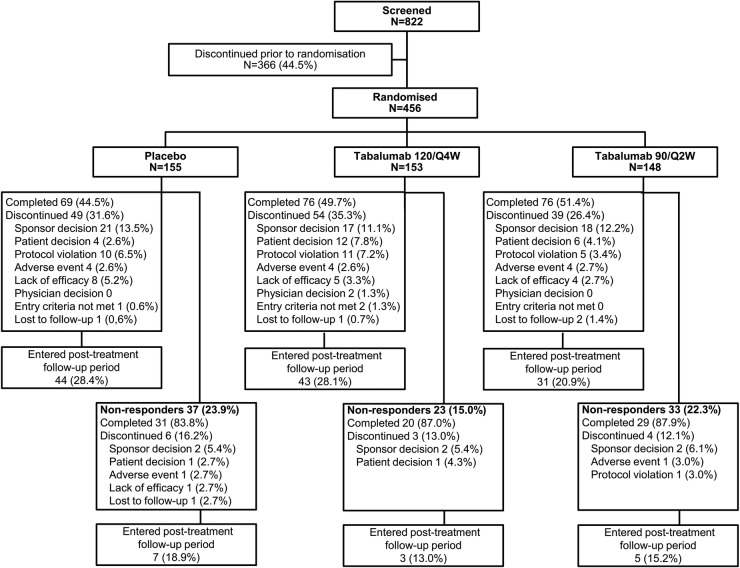
Figure 1.
Patient disposition. Eligibility was assessed during screening, then randomisation to 24 weeks of treatment (or 16 weeks for non-responders) in 1 of 2 tabalumab regimens or placebo and 48 weeks of follow-up. 120/Q4W=120 mg subcutaneous (SQ) tabalumab injection every 4 weeks; 90/Q2W=90 mg SQ tabalumab injection every 2 weeks.