Table 1.
Chemical structures of maritoclax derivatives bearing a pyoluteorin motif. Viability of U937 cells treated with the indicated compounds over 48 hours were used to calculate the EC50 values. For additional compounds that deviate from the pyoluteorin motif, see Table S1
| Pyoluteorin Motif | Compound | X | Y | Z | R1 | R2 | R3 | EC50 (μM) |
|---|---|---|---|---|---|---|---|---|
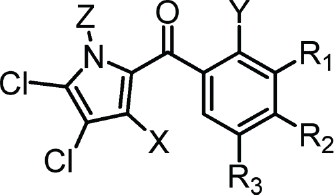 |
KS01 | ‒H | ‒OCH3 | ‒H | ‒H | ‒H | ‒H | > 50 |
| KS02 | ‒H | ‒OH | ‒H | ‒H | ‒H | ‒H | 19 | |
| KS03 | ‒Br | ‒OCH3 | ‒H | ‒H | ‒H | ‒H | > 50 | |
| KS04 | ‒Br | ‒OH | ‒H | ‒H | ‒H | ‒H | 1.7 | |
| KS05 | ‒Br | ‒OCH3 | ‒Br | ‒H | ‒H | ‒H | 13 | |
| KS06 | ‒Br | ‒OH | ‒Br | ‒H | ‒H | ‒H | 11 | |
| KS07 | ‒H | ‒OCH3 | ‒H | ‒OCH3 | ‒OCH3 | ‒H | > 50 | |
| KS08 | ‒H | ‒OH | ‒H | ‒OCH3 | ‒OCH3 | ‒H | 30 | |
| KS09 | ‒H | ‒OH | ‒H | ‒OH | ‒OH | ‒H | 33 | |
| KS11 | Br | ‒OCH3 | ‒H | ‒OCH3 | ‒OCH3 | ‒H | 22 | |
| KS12 | Br | ‒OH | ‒H | ‒OH | ‒OCH3 | ‒H | 5.0 | |
| KS13 | ‒CH3 | ‒OH | ‒H | ‒H | ‒H | ‒H | 13 | |
| KS14 | ‒Br | ‒OH | ‒H | ‒H | ‒OH | ‒H | 30 | |
| KS17 | ‒Br | ‒OCOCH3 | ‒H | ‒H | ‒H | ‒H | 1.8 | |
| KS18 | ‒Br | ‒OH | ‒H | ‒H | ‒H | ‒Cl | 0.5 | |
| KS18a | ‒Br | ‒OPO3H2 | ‒H | ‒H | ‒H | ‒Cl | 3.3 | |
| KS19 | ‒Br | ‒OCOCH3 | ‒H | ‒H | ‒H | ‒Cl | 0.5 | |
| KS20 | ‒Br | ‒OH | ‒H | ‒H | ‒H | ‒F | 0.7 | |
| KS21 | ‒Br | ‒OH | ‒H | ‒H | ‒H | ‒OH | 13 | |
| KS22 | ‒Br | ‒OH | ‒H | ‒H | ‒H | ‒CH3 | 3.1 | |
| KS23 | ‒Br | ‒OH | ‒H | ‒H | ‒H | ‒CH2CH3 | 2.5 | |
| KS24 | ‒Br | ‒OH | ‒H | ‒H | ‒H | ‒p‒PhCl | 0.6 | |
| KS27 | ‒Br | ‒OH | ‒H | ‒H | ‒H | ‒Br | 0.8 |
