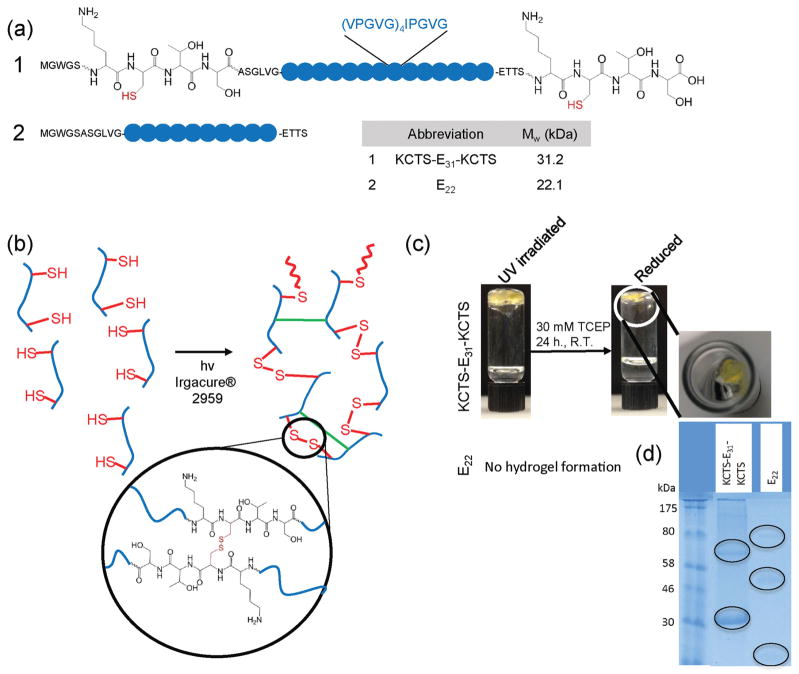
Figure 1.
Design and photocrosslinking of elastin-like polypeptides (ELPs). a) Sequences of expressed ELPs to test photocrosslinking. b) Proposed photocrosslinking mechanism for cysteine-containing ELP (KCTS-E31-KCTS), involving chain extension via disulfide bond formation (red bonds) and interchain crosslinking among ELP residues (green bonds). c) Representative images of 10 % (w/v) aqueous ELP solutions after UV exposure in the presence of a photoinitiator and after incubation with a reducing agent, tris (2-carboxyethyl) phosphine hydrochloride (TCEP). KCTS-E31-KCTS forms a gel after photocrosslinking while E22, an ELP lacking cysteine residues, remains a liquid. After reduction, KCTS-E31-KCTS is much smaller in size suggesting a mass loss after reduction. d) Protein electrophoresis gels of reduced hydrogels show bands (circled on gel), at double and triple the protein molecular weight when compared to nonreduced gels, indicating high molar mass proteins and the presence of some bonds other than disulfide bonds forming during photocrosslinking.