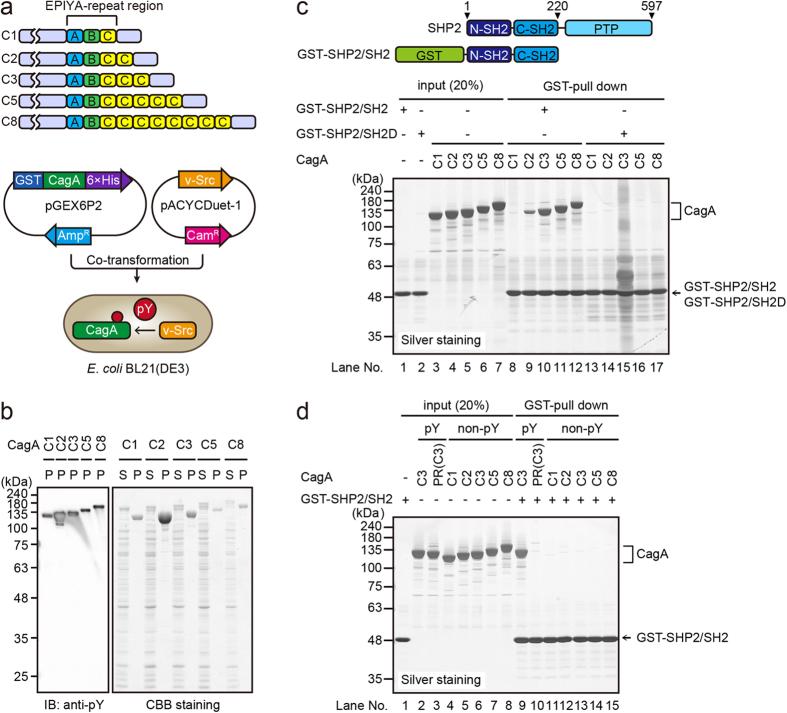
Figure 1. Tyrosine phosphorylation-dependent in vitro interaction of CagA with SHP2.
(a) Schematic representation of H. pylori CagA variants used in this work (upper). Strategy for tyrosine phosphorylation of recombinant CagA in E. coli (lower). E. coli BL21(DE3) was co-transformed with a GST-fused CagA expression vector and a v-Src expression vector. (b) Recombinant CagA proteins [CagA(Cn)-His] containing various numbers of EPIYA-C segments (C1, C2, C3, C5, and C8) were purified from E. coli co-expressing v-Src and subjected to immunoblotting with an anti-phosphotyrosine (pY) antibody (left) or CBB staining (right). S: supernatant of E. coli lysates containing GST-fused CagA, P: purified CagA after the cleavage of GST-tag. (c) GST pull-down assay was performed using tyrosine-phosphorylated CagA shown in (b) and GST-fused N-terminal SHP2 fragment containing two tandem-repeated SH2 domains, either functionally active (GST-SHP2/SH2) or dead (GST-SHP2/SH2D). CagA bound to GST-SHP2/SH2 was detected by silver staining. (d) GST pull-down assay was performed using GST-SHP2/SH2 and CagA containing three EPIYA-C segments (C3) or PR-CagA purified from E. coli expressing v-Src (lanes 1–3, 9, 10). GST pull-down assay was also performed using GST-SHP2/SH2 and CagA proteins containing various numbers of the EPIYA-C segments (C1, C2, C3, C5, C8) purified from E. coli without v-Src expression (lanes 4–8, 11–15). CagA bound to GST-SHP2/SH2 was detected by silver staining.