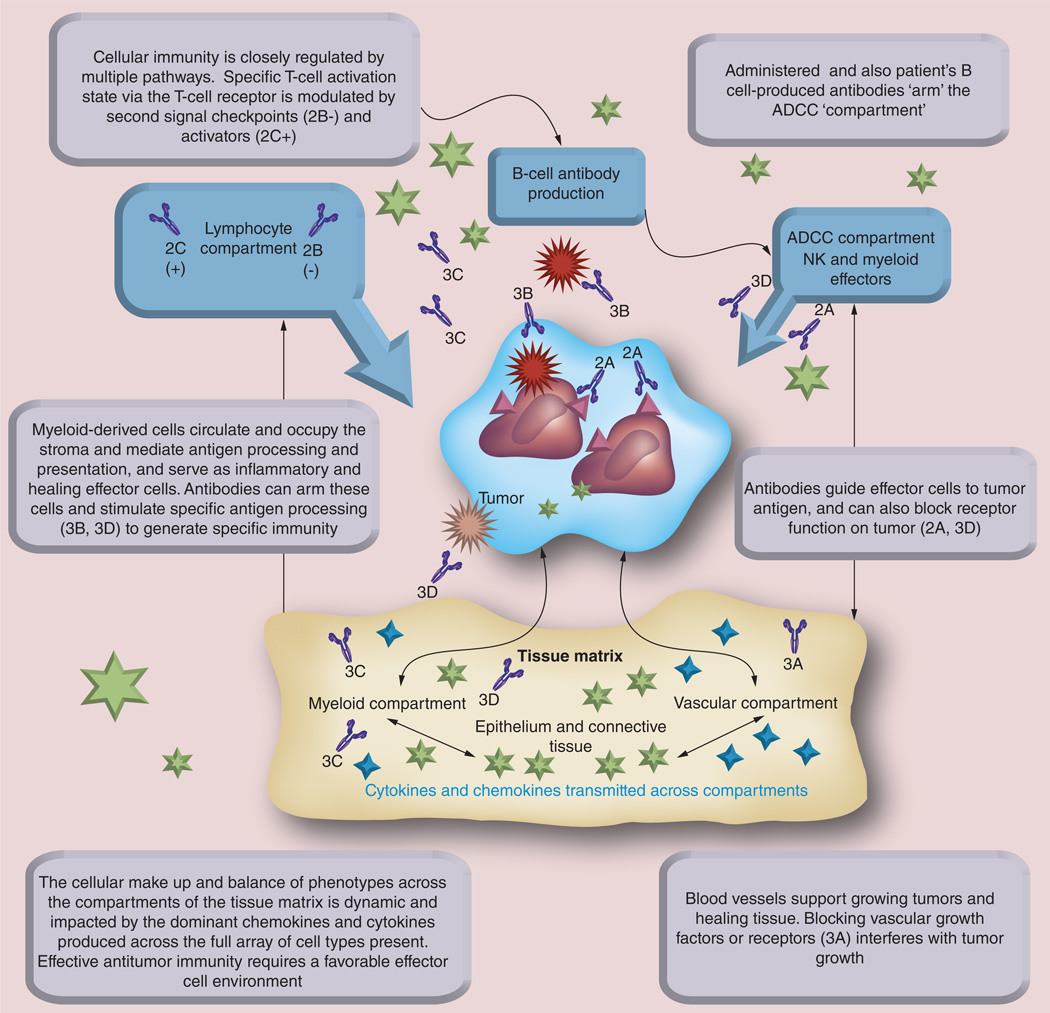
Figure 1. The balance of host–tumor interaction is defined by the tissue matrix in which a tumor is growing.
Antibodies not only arm effector cells such as NK cells and Fc receptor-bearing cells for the IgG and IgE classes to give them specificity in function, but can directly interfere with receptor-mediated tumor cell growth and activation (2A). By targeting second signal interactions between lymphocytes and both antigen processing cells and the lymphocyte targets, specific antibodies can serve to enhance T-cell immunity by blocking checkpoint down regulation (2B) and by stimulating second-signal activators (2C). Vascular support of the tumor microenvironment can be reduced by antibodies to vascular growth factors and their receptors on growing microvasculature, thus limiting tumor growth and nutrition (3A). Antibodies to tumor antigen can selectively enhance antigen presentation and emergence of tumor-specific immunity (3B). Antibody neutralization of specific chemokine and cytokine activity (3C) can further modulate the balance of a tumor microenvironment to favor tumor-eliminating immunity. Monoclonal IgE targeting tumor antigens (3D) can enhance tumor antigen processing and T-cell immunity and also arm myeloid effector cells to target tumor cells in the tissue stroma. Drugs of multiple classes can also influence these interactions, such that optimal cancer therapy is multimodality and should be crafted to modulate the tumor environment to favor growth impairment, cytotoxicity and immune-mediated tumor destruction. Antibody products labelled 2A–C and 3A–D correspond with Table 2A–C and Table 3A–D, where each antibody product is described in-depth.