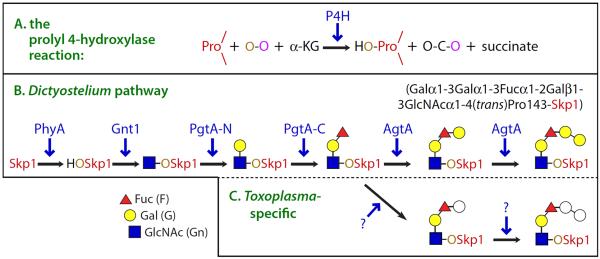
Fig. 1.
The Skp1 modification pathway in Dictyostelium and Toxoplasma. (A) Summary of the reaction catalyzed by the P4H class of non-heme dioxygenases, in which a protein-linked Pro residues is subject to hydroxylation at its 4-position by an O-atom derived from O2 (O-O). The other O-atom is transferred to α-KG, which decomposes into CO2 and succinate. (B) The hydroxyl group generated by the action of the P4H PhyA on Skp1 provides the site for glycosylation by five glycosyltransferase activities encoded by 3 genes in Dictyostelium. (C) Toxoplasma also assembles a pentasaccharide with the same Hex-Hex-dHex-Hex-HexNAc– composition (unpublished data). Tg-PhyA mediates Skp1 hydroxylation and is required for glycosylation, which has been confirmed by mass spectrometry (in preparation). gnt1 and pgtA are conserved in Toxoplasma, and likely modify Toxoplasma Skp1 as they do in Dictyostelium. However, the physical order of the N- and C-terminal catalytic domains of Dd-PgtA, which mediate the addition of β-Gal and α-Fuc, respectively, is reversed in Toxoplasma.