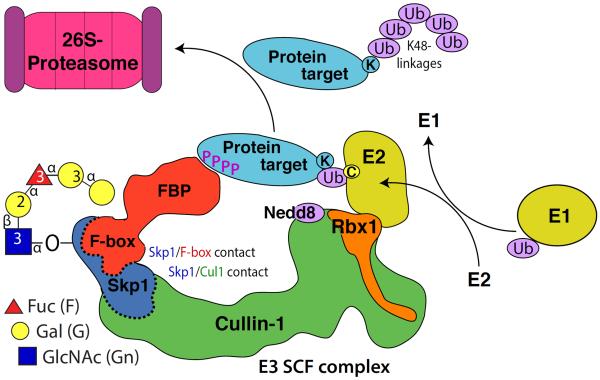
Fig. 2.
Skp1 hydroxylation and glycosylation likely regulate the SCF complex, an E3 ubiquitin ligase that marks target proteins with K48-linked polyubiquitin chains that are recognized by the proteasome for degradation. Polyubiquitination involves successive transfers of Ub from Ub-E2 donors that cycle on, off, and on again after recharging from Ub-E1. The process is controlled by, in some cases, substrate priming such as phosphorylation (P=PO4) as indicated. Ubiquitination is also regulated by neddylation of Cul1, which is under the control of the COP9 signalosome and other factors and promotes flexible tethering of Rbx1 and its associated Ub-E2 to allow access to the Ub target site (K=Lys) on the substrate. Neddylation also excludes Cand1 (not shown), which allows docking of FBP/Skp1 complexes to Cul1 (dashed interface). Skp1 modifications, which occur near its C-terminus, are proposed to promote interactions of FBPs with Skp1 (dashed interface), providing novel SCF-specific regulation. Modified from [42].