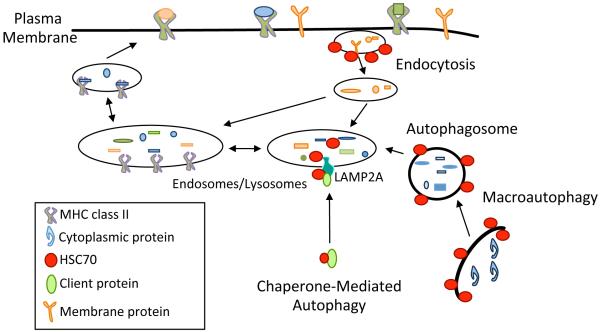
Figure 1. The role of HSC70 in MHC class II antigen trafficking and presentation.
Exogenous Ags are internalized via clathrin-mediated endocytosis and delivered to the endosomal/lysosomal network. Endogenous Ags can be delivered by autophagy pathways such as macroautophagy (MA) and chaperone-mediated autophagy (CMA). Once delivered into the endosomal/lysosomal network, acidic proteases within these compartments process the Ag into small peptides. These peptides then bind MHC class II molecules. MHC class II:peptide complexes are shuttled to the surface to be displayed for recognition by CD4+ T cells. HSC70 is required for clathrin-mediated endocytosis, as the ATPase activity of HSC70 is required for coating and uncoating of vesicles. HSC70 is a key component of CMA, binding to client proteins and guiding them to the lysosomal membrane for interactions with LAMP2A. Furthermore, HSC70 in the lumen of the lysosome is required for translocation of these peptides. While association of HSC70 with autophagsome membranes has been detected, the function of this has not been established though it may be related to selective-autophagy. During selective-autophagy, HSC70 and its co-chaperone BAG3 guide client proteins to p62 and LC3, leading to their degradation via MA.