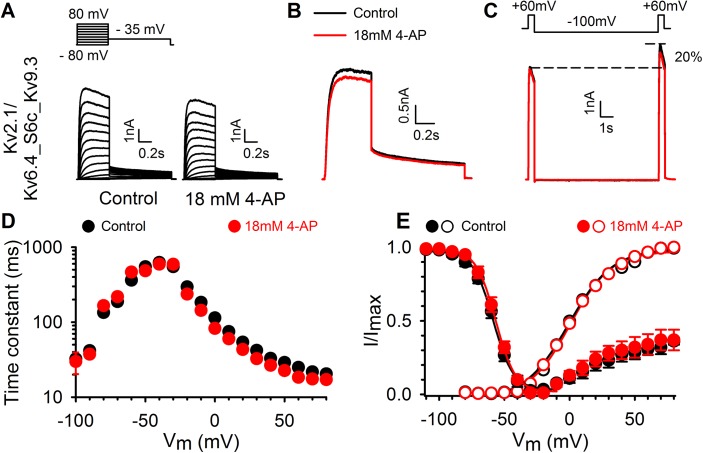
Fig 6. Involvement of the S6c domain in the KvS specific response to 4−AP.
(A) Typical current traces for the Kv2.1/Kv6.4_S6c_Kv9.3 chimera without (left) and with 4−AP (right) revealing inhibition by 4−AP, comparable to its effect on Kv2.1/Kv9.3_WT currents. The pulse protocol is shown above the current traces. (B) 4−AP (red trace) causes a small inhibiting effect of Kv2.1/Kv6.4_S6c_Kv9.3 currents which is the opposite of the 4−AP effect on the Kv2.1/Kv6.4_WT currents. Control trace shown in black. (C) The twin pulse protocol from Fig 4E was repeated on the Kv6.4_S6c_Kv9.3 chimera to illustrate that: 1) closed−state inactivation is still present at −80mV, 2) inactivated channels can be recovered in a 10s pulse to −100 mV and 3) 4−AP is unable to interfere with the closed−state inactivation of the Kv6.4_S6c_Kv9.3 chimera. (D) Activation and deactivation kinetics of the Kv2.1/Kv6.4_S6c_Kv9.3 heterotetramers (black circles). Time constants of the chimera were obtained with a single exponential function which resulted in a single fast component that remained unaltered under the influence of 4−AP (red circles). This chimera lacked the pronounced slow component present in Kv2.1/Kv6.4_WT heterotetramers (Fig 3C). (E) Voltage−dependence of activation (open symbols) and inactivation (closed symbols) of the Kv2.1/Kv6.4_S6c_Kv9.3 chimera obtained like for the WT Kv2.1/Kv6.4 heterotetramers in Fig 3. Both were nearly the same as for WT Kv2.1/Kv6.4 currents and remained unaffected by 4−AP application (red symbols).