Figure 3.
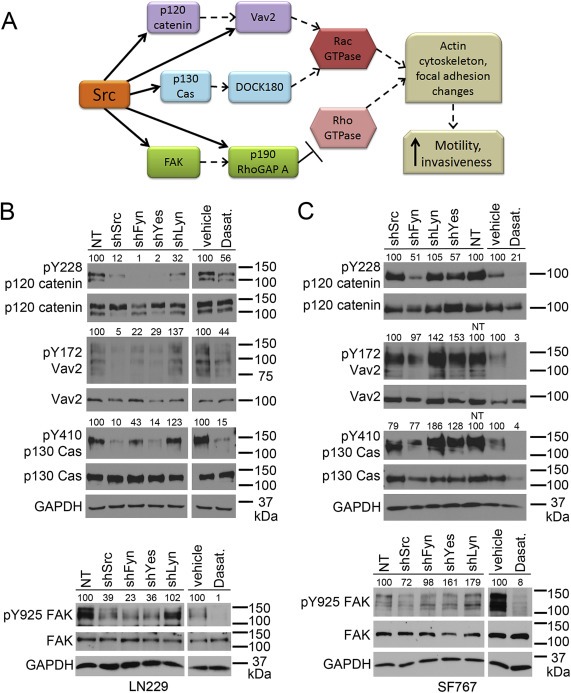
Src‐family kinases differentially target motility‐related proteins. (A) SFKs phosphorylate multiple proteins upstream of the Rac and Rho GTPases, leading to actin cytoskeleton and focal adhesion changes that increase cell motility and invasiveness. In particular, SFKs directly phosphorylate (solid arrows) Y228 on p120 catenin, Y410 on p130 Cas, Y172 on Vav2 (which is also regulated (dashed arrow) by p120 catenin), Y925 on FAK, and Y1105 on p190 RhoGAP A (which is also regulated by FAK). (B,C) Western blot evaluation of motility‐related protein phosphorylation in LN229 (B) and SF767 (C) cells. Lysates were made from cells expressing NT or individual SFK shRNAs, or treated with DMSO vehicle or 10 μM dasatinib for 17–24 h (17–18.5 for LN229, 24 for SF767). PhosphoY228 and total p120 catenin, phosphoY172 and total Vav2, phosphoY410 and total p130 Cas, and phosphoY925 and total FAK were evaluated; GAPDH is the loading control. Numbers above each of the phospho‐protein blots indicate expression relative to the NT lysate (% of NT for NT and shSFK lysates) or the vehicle lysate (% of vehicle for vehicle and dasatinib lysates) and are normalized to the corresponding total protein level as well as to GAPDH expression.
